Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia
- PMID: 23831595
- PMCID: PMC3831035
- DOI: 10.1038/mt.2013.154
Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia
Abstract (VSports)
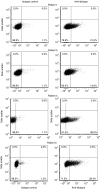
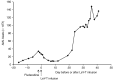
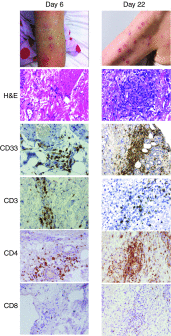
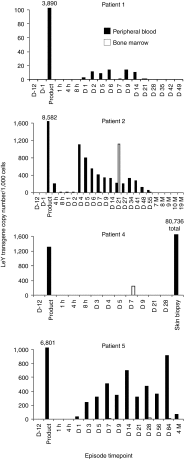
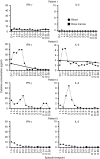
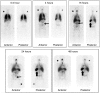
In a phase I study of autologous chimeric antigen receptor (CAR) anti-LeY T-cell therapy of acute myeloid leukemia (AML), we examined the safety and postinfusion persistence of adoptively transferred T cells. Following fludarabine-containing preconditioning, four patients received up to 1. 3 × 109 total T cells, of which 14-38% expressed the CAR. Grade 3 or 4 toxicity was not observed. One patient achieved a cytogenetic remission whereas another with active leukemia had a reduction in peripheral blood (PB) blasts and a third showed a protracted remission. Using an aliquot of In111-labeled CAR T cells, we demonstrated trafficking to the bone marrow (BM) in those patients with the greatest clinical benefit. Furthermore, in a patient with leukemia cutis, CAR T cells infiltrated proven sites of disease. Serial PCR of PB and BM for the LeY transgene demonstrated that infused CAR T cells persisted for up to 10 months. Our study supports the feasibility and safety of CAR-T-cell therapy in high-risk AML, and demonstrates durable in vivo persistence. VSports手机版.
Figures
Comment in
-
CAR T cells for acute myeloid leukemia: the LeY of the land.Mol Ther. 2013 Nov;21(11):1983-4. doi: 10.1038/mt.2013.234. Mol Ther. 2013. PMID: 24201214 Free PMC article. No abstract available.
References
-
- Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117:2307–2318. - "V体育官网入口" PubMed
-
- Wen YJ, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001;97:1750–1755. - PubMed
-
- Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–3285. - PubMed
-
- Parker LL, Do MT, Westwood JA, Wunderlich JR, Dudley ME, Rosenberg SA, et al. Expansion and characterization of T cells transduced with a chimeric receptor against ovarian cancer. Hum Gene Ther. 2000;11:2377–2387. - PubMed (VSports app下载)
Publication types
"VSports app下载" MeSH terms
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- Actions (VSports app下载)
- "VSports" Actions
- Actions (V体育安卓版)
- "VSports" Actions
- V体育ios版 - Actions
- "V体育官网入口" Actions
- "VSports最新版本" Actions
- Actions (VSports在线直播)
"V体育官网" Substances
- V体育官网入口 - Actions
- "V体育ios版" Actions
V体育2025版 - LinkOut - more resources
"VSports注册入口" Full Text Sources
Other Literature Sources
"V体育ios版" Medical

