Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration
- PMID: 23814022
- PMCID: PMC3778553
- DOI: 10.1182/blood-2012-11-470252
Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration
Abstract
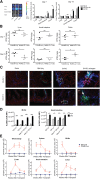
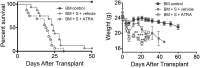
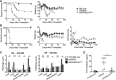
Graft-versus-host disease (GVHD) is a critical complication after allogeneic bone marrow transplantation. During GVHD, donor T cells are activated by host antigen-presenting cells and differentiate into T-effector cells (Teffs) that migrate to GVHD target organs. However, local environmental factors influencing Teff differentiation and migration are largely unknown. Vitamin A metabolism within the intestine produces retinoic acid, which contributes to intestinal homeostasis and tolerance induction. Here, we show that the expression and function of vitamin A-metabolizing enzymes were increased in the intestine and mesenteric lymph nodes in mice with active GVHD. Moreover, transgenic donor T cells expressing a retinoic acid receptor (RAR) response element luciferase reporter responded to increased vitamin A metabolites in GVHD-affected organs. Increasing RAR signaling accelerated GVHD lethality, whereas donor T cells expressing a dominant-negative RARα (dnRARα) showed markedly diminished lethality. The dnRARα transgenic T cells showed reduced Th1 differentiation and α4β7 and CCR9 expression associated with poor intestinal migration, low GVHD pathology, and reduced intestinal permeability, primarily via CD4(+) T cells. The inhibition of RAR signaling augmented donor-induced Treg generation and expansion in vivo, while preserving graft-versus-leukemia effects VSports手机版. Together, these results suggested that reagents blunting donor T-cell RAR signaling may possess therapeutic anti-GVHD properties. .
Figures (VSports在线直播)



References
-
- Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. - PubMed
-
- Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–3213. - PubMed
-
- Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8(6):575–581. - PubMed
-
- Takashima S, Kadowaki M, Aoyama K, et al. The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208(2):285–294. - VSports手机版 - PMC - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- Actions (VSports)
- "VSports" Actions
- VSports app下载 - Actions
- "V体育安卓版" Actions
- VSports手机版 - Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- "V体育ios版" Actions
Substances
- "VSports手机版" Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
- Actions (V体育平台登录)
Grants and funding
- R01 HL115761/HL/NHLBI NIH HHS/United States (V体育安卓版)
- V体育官网入口 - P30 CA016086/CA/NCI NIH HHS/United States
- V体育安卓版 - MR/J006742/1/MRC_/Medical Research Council/United Kingdom
- R01 CA062275/CA/NCI NIH HHS/United States
- 091823/WT_/Wellcome Trust/United Kingdom
- R01 AI034495/AI/NIAID NIH HHS/United States
- R01 AI34495/AI/NIAID NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States (V体育平台登录)
- R01 CA072669/CA/NCI NIH HHS/United States
- V体育ios版 - P01 CA142106/CA/NCI NIH HHS/United States
- R01-CA062275/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- R01 AT005382/AT/NCCIH NIH HHS/United States
- R01 HL56067/HL/NHLBI NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States (V体育平台登录)
- P30 CA023108/CA/NCI NIH HHS/United States
- G0802651/MRC_/Medical Research Council/United Kingdom (V体育平台登录)
- F32 AI010302/AI/NIAID NIH HHS/United States
- R01 CA166794/CA/NCI NIH HHS/United States
"VSports手机版" LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育ios版 - Research Materials

