C/EBPγ suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPβ
- PMID: 23775115
- PMCID: PMC3753923
- DOI: 10.1128/MCB.01674-12 (V体育官网入口)
VSports手机版 - C/EBPγ suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPβ
Abstract
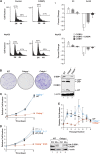
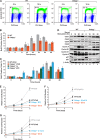
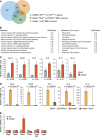
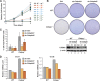
C/EBPβ is an important regulator of oncogene-induced senescence (OIS). Here, we show that C/EBPγ, a heterodimeric partner of C/EBPβ whose biological functions are not well understood, inhibits cellular senescence VSports手机版. Cebpg(-/-) mouse embryonic fibroblasts (MEFs) proliferated poorly, entered senescence prematurely, and expressed a proinflammatory gene signature, including elevated levels of senescence-associated secretory phenotype (SASP) genes whose induction by oncogenic stress requires C/EBPβ. The senescence-suppressing activity of C/EBPγ required its ability to heterodimerize with C/EBPβ. Covalently linked C/EBPβ homodimers (β∼β) inhibited the proliferation and tumorigenicity of Ras(V12)-transformed NIH 3T3 cells, activated SASP gene expression, and recruited the CBP coactivator in a Ras-dependent manner, whereas γ∼β heterodimers lacked these capabilities and efficiently rescued proliferation of Cebpg(-/-) MEFs. C/EBPβ depletion partially restored growth of C/EBPγ-deficient cells, indicating that the increased levels of C/EBPβ homodimers in Cebpg(-/-) MEFs inhibit proliferation. The proliferative functions of C/EBPγ are not restricted to fibroblasts, as hematopoietic progenitors from Cebpg(-/-) bone marrow also displayed impaired growth. Furthermore, high CEBPG expression correlated with poorer clinical prognoses in several human cancers, and C/EBPγ depletion decreased proliferation and induced senescence in lung tumor cells. Our findings demonstrate that C/EBPγ neutralizes the cytostatic activity of C/EBPβ through heterodimerization, which prevents senescence and suppresses basal transcription of SASP genes. .
Figures





References
-
- Campisi J. 2005. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522 - PubMed
-
- Lowe SW, Cepero E, Evan G. 2004. Intrinsic tumour suppression. Nature 432:307–315 - PubMed
-
- Coppe J-P, Desprez P-Y, Krtolica A, Campisi J. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5:99–118 - "VSports手机版" PMC - PubMed
-
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, Iken M, Vucur M, Weiss S, Heikenwalder M, Khan S, Gil J, Bruder D, Manns M, Schirmacher P, Tacke F, Ott M, Luedde T, Longerich T, Kubicka S, Zender L. 2011. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479:547–551 - PubMed (VSports在线直播)
Publication types
- "VSports最新版本" Actions
V体育2025版 - MeSH terms
- "V体育2025版" Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions
- VSports app下载 - Actions
- VSports app下载 - Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- Actions (V体育官网)
- Actions (V体育安卓版)
- Actions (V体育2025版)
Substances
- Actions (VSports)
- VSports手机版 - Actions
VSports注册入口 - Grants and funding
"VSports" LinkOut - more resources
VSports在线直播 - Full Text Sources
Other Literature Sources
Molecular Biology Databases
