Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors
- PMID: 23734311
- PMCID: PMC3654581
- DOI: 10.4161/onci.23564
Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors
Abstract
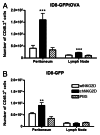
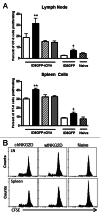
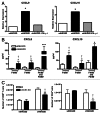
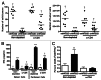
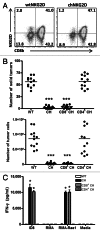
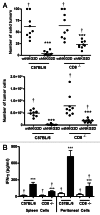
Conditioning strategies that deplete host lymphocytes have been shown to enhance clinical responses to some adoptive T-cell therapies. However, host T cells are capable of eliminating tumor cells upon the relief of immunosuppression, indicating that lymphodepletion prior to T-cell transfer may reduce optimal tumor protection elicited by cell treatments that are capable of shaping host immunity. In this study, we show that adoptively transferred T cells bearing a chimeric antigen receptor (CAR) harness endogenous T cells for optimal tumor elimination and the development of a tumor-specific memory T cell response. Mice bearing ID8 ovarian cancer cells were treated with T cells transduced with a NKG2D-based CAR. CAR-expressing T cells increased the number of host CD4+ and CD8+ T cells at the tumor site in a CXCR3-dependent manner and increased the number of antigen-specific host CD4+ T cells in the tumor and draining lymph nodes. In addition, the administration of CAR-expressing T cells increased antigen presentation to CD4+ T cells, and this increase was dependent on interferon γ and granulocyte-macrophage colony-stimulating factor produced by the former. Host CD4+ T cells were sufficient for optimal tumor protection mediated by NKG2D CAR-expressing T cells, but they were not necessary if CD4+ T cells were adoptively co-transferred VSports手机版. However, host CD4+ T cells were essential for the development of an antigen-specific memory T-cell response to tumor cells. Moreover, optimal tumor elimination as orchestrated by NKG2D CAR-expressing T cells was dependent on host CD8+ T cells. These results demonstrate that adoptively transferred T cells recruit and activate endogenous T-cell immunity to enhance the elimination of tumor cells and the development of tumor-specific memory responses. .
Keywords: CD4+ T cells; CD8+ T cells; ID8; NKG2D; adoptive T cell therapy; chNKG2D; immunotherapy; ovarian cancer; tumor immunology V体育安卓版. .
Figures
References
-
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. - "VSports" DOI - PMC - PubMed
-
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. - DOI - PMC - PubMed
-
- el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, et al. MHC Class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29:3295–301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. - DOI - PubMed
"V体育安卓版" Publication types
- VSports - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
VSports最新版本 - Other Literature Sources
V体育官网入口 - Research Materials
