Caspase-3 is involved in the signalling in erythroid differentiation by targeting late progenitors
- PMID: 23658722
- PMCID: VSports app下载 - PMC3642196
- DOI: 10.1371/journal.pone.0062303
Caspase-3 is involved in the signalling in erythroid differentiation by targeting late progenitors
Abstract (VSports手机版)
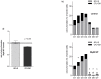
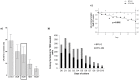
A role for caspase activation in erythroid differentiation has been established, yet its precise mode of action remains elusive. A drawback of all previous investigations on caspase activation in ex vivo erythroid differentiation is the lack of an in vitro model producing full enucleation of erythroid cells. Using a culture system which renders nearly 100% enucleated red cells from human CD34(+) cells, we investigated the role of active caspase-3 in erythropoiesis. Profound effects of caspase-3 inhibition were found on erythroid cell growth and differentiation when inhibitors were added to CD34(+) cells at the start of the culture and showed dose-response to the concentration of inhibitor employed VSports手机版. Enucleation was only reduced as a function of the reduced maturity of the culture and the increased cell death of mature cells while the majority of cells retained their ability to extrude their nuclei. Cell cycle analysis after caspase-3 inhibition showed caspase-3 to play a critical role in cell proliferation and highlighted a novel function of this protease in erythroid differentiation, i. e. its contribution to cell cycle regulation at the mitotic phase. While the effect of caspase-3 inhibitor treatment on CD34(+) derived cells was not specific to the erythroid lineage, showing a similar reduction of cell expansion in myeloid cultures, the mechanism of action in both lineages appeared to be distinct with a strong induction of apoptosis causing the decreased yield of myeloid cells. Using a series of colony-forming assays we were able to pinpoint the stage at which cells were most sensitive to caspase-3 inhibition and found activated caspase-3 to play a signalling role in erythroid differentiation by targeting mature BFU-E and CFU-E but not early BFU-E. .
Conflict of interest statement
Figures




References
-
- Chowdhury I, Tharakan B, Bhat GK (2008) Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol 151: 10–27. - PubMed
-
- Feinstein-Rotkopf Y, Arama E (2009) Can't live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis 14: 980–995. - PubMed
-
- Carlile GW, Smith DH, Wiedmann M (2004) Caspase-3 has a nonapoptotic function in erythroid maturation. Blood 103: 4310–4316. - PubMed
Publication types
MeSH terms (VSports)
- Actions (V体育平台登录)
- Actions (V体育官网)
- "V体育官网" Actions
- "VSports" Actions
- Actions (V体育官网)
- V体育官网入口 - Actions
- Actions (V体育ios版)
- Actions (V体育官网)
- Actions (VSports)
Substances
- VSports注册入口 - Actions
LinkOut - more resources
V体育2025版 - Full Text Sources
Other Literature Sources
Research Materials

