Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity (VSports)
- PMID: 23597565
- PMCID: PMC3658176
- DOI: 10.1016/j.ccr.2013.03.018
"V体育官网" Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity
Abstract (VSports app下载)
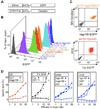
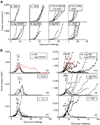
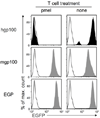
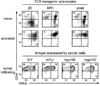
Cancers often relapse after adoptive therapy, even though specific T cells kill cells from the same cancer efficiently in vitro VSports手机版. We found that tumor eradication by T cells required high affinities of the targeted peptides for major histocompatibility complex (MHC) class I. Affinities of at least 10 nM were required for relapse-free regression. Only high-affinity peptide-MHC interactions led to efficient cross-presentation of antigen, thereby stimulating cognate T cells to secrete cytokines. These findings highlight the importance of targeting peptides with high affinity for MHC class I when designing T cell-based immunotherapy. .
Copyright © 2013 Elsevier Inc. All rights reserved V体育安卓版. .
Conflict of interest statement
The authors have no conflict of interests.
Figures
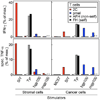
Comment in
-
It's the peptide-MHC affinity, stupid. (VSports最新版本)Cancer Cell. 2013 Apr 15;23(4):429-31. doi: 10.1016/j.ccr.2013.04.004. Cancer Cell. 2013. PMID: 23597560
References
-
- Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. - PMC - PubMed
-
- Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui HH, Frahm N, Brander C, Peters B, Grey H, Sette A. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol. 2007;178:7890–7901. - PubMed
-
- Bowerman NA, Colf LA, Garcia KC, Kranz DM. Different strategies adopted by K(b) and L(d) to generate T cell specificity directed against their respective bound peptides. J Biol Chem. 2009;284:32551–32561. - PMC (V体育2025版) - PubMed
-
- Budhu S, Loike JD, Pandolfi A, Han S, Catalano G, Constantinescu A, Clynes R, Silverstein SC. CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues. J Exp Med. 2010;207:223–235. - V体育安卓版 - PMC - PubMed
VSports最新版本 - Publication types
MeSH terms
- V体育安卓版 - Actions
- VSports在线直播 - Actions
- V体育平台登录 - Actions
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- Actions (V体育官网)
Substances
- VSports注册入口 - Actions
- VSports手机版 - Actions
Grants and funding (V体育平台登录)
LinkOut - more resources
"VSports注册入口" Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

