Apoptosis and other immune biomarkers predict influenza vaccine responsiveness
- PMID: 23591775
- PMCID: PMC3658270
- DOI: 10.1038/msb.2013.15
Apoptosis and other immune biomarkers predict influenza vaccine responsiveness
Erratum in
- Mol Syst Biol. 2013;9:680
- Mol Syst Biol. 2014;10:750
V体育安卓版 - Abstract
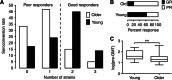
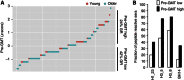
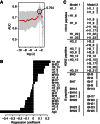
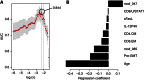
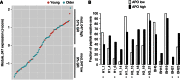
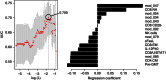
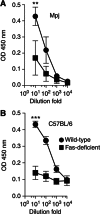
Despite the importance of the immune system in many diseases, there are currently no objective benchmarks of immunological health. In an effort to identifying such markers, we used influenza vaccination in 30 young (20-30 years) and 59 older subjects (60 to >89 years) as models for strong and weak immune responses, respectively, and assayed their serological responses to influenza strains as well as a wide variety of other parameters, including gene expression, antibodies to hemagglutinin peptides, serum cytokines, cell subset phenotypes and in vitro cytokine stimulation. Using machine learning, we identified nine variables that predict the antibody response with 84% accuracy. Two of these variables are involved in apoptosis, which positively associated with the response to vaccination and was confirmed to be a contributor to vaccine responsiveness in mice. The identification of these biomarkers provides new insights into what immune features may be most important for immune health. VSports手机版.
Conflict of interest statement (V体育安卓版)
The authors declare that they have no conflict of interest.
Figures

References
-
- Akaike H (1974) A new look at the statistical model identification. IEEE Trans Auto Control 19: 716–723
-
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B-57: 289–300
-
- Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD (1999) Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med 159: 182–188 - VSports最新版本 - PubMed
-
- Beyer WE, Palache AM, Luchters G, Nauta J, Osterhaus AD (2004) Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res 103: 125–132 - PubMed
Publication types
MeSH terms
- Actions (V体育安卓版)
- VSports最新版本 - Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
- "V体育安卓版" Actions
- Actions (VSports手机版)
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "VSports在线直播" Actions
- V体育官网 - Actions
- Actions (V体育官网)
- "VSports最新版本" Actions
- VSports在线直播 - Actions
- "V体育官网入口" Actions
- "VSports" Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- Actions (V体育2025版)
- V体育官网入口 - Actions
Substances
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (V体育安卓版)

