V体育ios版 - Lessons learned and unlearned in periodontal microbiology
- PMID: 23574465
- PMCID: PMC3912758
- DOI: 10.1111/prd.12010
Lessons learned and unlearned in periodontal microbiology
V体育官网 - Abstract
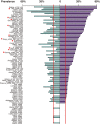
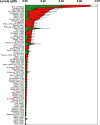
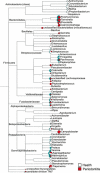
Periodontal diseases are initiated by bacterial species living in polymicrobial biofilms at or below the gingival margin and progress largely as a result of the inflammation elicited by specific subgingival species. In the past few decades, efforts to understand the periodontal microbiota have led to an exponential increase in information about biofilms associated with periodontal health and disease. In fact, the oral microbiota is one of the best-characterized microbiomes that colonize the human body. Despite this increased knowledge, one has to ask if our fundamental concepts of the etiology and pathogenesis of periodontal diseases have really changed VSports手机版. In this article we will review how our comprehension of the structure and function of the subgingival microbiota has evolved over the years in search of lessons learned and unlearned in periodontal microbiology. More specifically, this review focuses on: (i) how the data obtained through molecular techniques have impacted our knowledge of the etiology of periodontal infections; (ii) the potential role of viruses in the etiopathogenesis of periodontal diseases; (iii) how concepts of microbial ecology have expanded our understanding of host-microbe interactions that might lead to periodontal diseases; (iv) the role of inflammation in the pathogenesis of periodontal diseases; and (v) the impact of these evolving concepts on therapeutic and preventive strategies to periodontal infections. We will conclude by reviewing how novel systems-biology approaches promise to unravel new details of the pathogenesis of periodontal diseases and hopefully lead to a better understanding of their mechanisms. .
© 2013 John Wiley & Sons A/S.
Figures



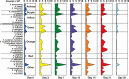


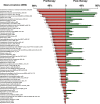
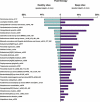
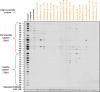





V体育2025版 - References
-
- Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol 1996: 1: 926–932. - PubMed
-
- Proceedings of the 1996 World Workshop in Periodontics. Lansdowne, Virginia, July 13–17, 1996. Ann Periodontol 1996: 1: 1–947. - PubMed
-
- Abiko Y, Sato T, Mayanagi G, Takahashi N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real‐time PCR. J Periodontal Res 2010: 45: 389–395. - PubMed
-
- Abramson JS, Mills EL. Depression of neutrophil function induced by viruses and its role in secondary microbial infections. Rev Infect Dis 1988: 10: 326–341. - PubMed
Publication types
MeSH terms
- Actions (VSports)
- "VSports app下载" Actions
- "VSports app下载" Actions
- Actions (VSports)
Grants and funding
LinkOut - more resources
VSports - Full Text Sources
Other Literature Sources
Miscellaneous

