Atrx deficiency induces telomere dysfunction, endocrine defects, and reduced life span
- PMID: 23563309
- PMCID: "VSports手机版" PMC3635723
- DOI: 10.1172/JCI65634
Atrx deficiency induces telomere dysfunction, endocrine defects, and reduced life span
Abstract
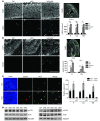
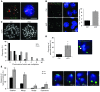
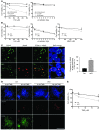
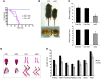
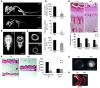
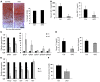
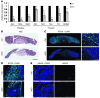
Human ATRX mutations are associated with cognitive deficits, developmental abnormalities, and cancer. We show that the Atrx-null embryonic mouse brain accumulates replicative damage at telomeres and pericentromeric heterochromatin, which is exacerbated by loss of p53 and linked to ATM activation. ATRX-deficient neuroprogenitors exhibited higher incidence of telomere fusions and increased sensitivity to replication stress-inducing drugs VSports手机版. Treatment of Atrx-null neuroprogenitors with the G-quadruplex (G4) ligand telomestatin increased DNA damage, indicating that ATRX likely aids in the replication of telomeric G4-DNA structures. Unexpectedly, mutant mice displayed reduced growth, shortened life span, lordokyphosis, cataracts, heart enlargement, and hypoglycemia, as well as reduction of mineral bone density, trabecular bone content, and subcutaneous fat. We show that a subset of these defects can be attributed to loss of ATRX in the embryonic anterior pituitary that resulted in low circulating levels of thyroxine and IGF-1. Our findings suggest that loss of ATRX increases DNA damage locally in the forebrain and anterior pituitary and causes tissue attrition and other systemic defects similar to those seen in aging. .
Figures
References
"V体育官网入口" Publication types
- "V体育官网" Actions
MeSH terms
- Actions (V体育官网入口)
- "V体育2025版" Actions
- Actions (V体育2025版)
- Actions (VSports注册入口)
- Actions (VSports手机版)
- V体育平台登录 - Actions
- "VSports最新版本" Actions
- "V体育ios版" Actions
- VSports手机版 - Actions
- "VSports" Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- "V体育2025版" Actions
- Actions (VSports手机版)
- "VSports注册入口" Actions
- Actions (V体育官网)
- Actions (V体育官网入口)
- "V体育平台登录" Actions
"V体育平台登录" Substances
- Actions (VSports注册入口)
- VSports app下载 - Actions
- "VSports手机版" Actions
- "VSports在线直播" Actions
- Actions (V体育官网入口)
"VSports" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports最新版本 - Research Materials
Miscellaneous

