Liver-resident NK cells confer adaptive immunity in skin-contact inflammation
- PMID: 23524967
- PMCID: PMC3613925 (VSports注册入口)
- DOI: "V体育安卓版" 10.1172/JCI66381
Liver-resident NK cells confer adaptive immunity in skin-contact inflammation
Abstract
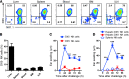
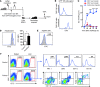
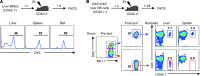
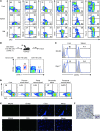
Liver natural killer (NK) cells were recently reported to possess memory-like properties in contact hypersensitivity (CHS) models. However, the phenotype and origin of these "memory" NK cells cannot be distinguished from other NK cell subpopulations. Here, we define the transcriptional, phenotypic, and functional features of liver NK cell subsets and their roles in mediating CHS. Liver NK cells can be divided into two distinct subsets: CD49a+DX5- and CD49a-DX5+. Substantial transcriptional and phenotypic differences existed between liver CD49a+DX5- NK cells and other NK cell subsets. CD49a+DX5- NK cells possessed memory potential and conferred hapten-specific CHS responses upon hapten challenge VSports手机版. Importantly, CD49a+DX5- NK cells were liver resident and were present in the liver sinusoidal blood, but not the afferent and efferent blood of the liver. Moreover, they appeared to originate from hepatic hematopoietic progenitor/stem cells (HPCs/HSCs) but not from the bone marrow, and maintained their phenotypes in the steady state. Our findings of liver-resident NK cells shed new light on the acquisition of memory-like properties of NK cells. .
Figures (V体育2025版)

VSports注册入口 - References
-
- Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–531. doi: 10.1038/nri1863. - V体育ios版 - DOI - PubMed
-
- Seaman WE, Blackman MA, Gindhart TD, Roubinian JR, Loeb JM, Talal N. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978;121(6):2193–2198. - PubMed
-
- Kumar V, Ben-Ezra J, Bennett M, Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979;123(4):1832–1838. - PubMed
Publication types
MeSH terms
- V体育官网 - Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- Actions (VSports)
- V体育ios版 - Actions
- Actions (V体育官网)
- "V体育ios版" Actions
- Actions (V体育2025版)
- Actions (VSports app下载)
Substances (V体育官网入口)
- VSports注册入口 - Actions
- Actions (V体育官网入口)
Grants and funding
LinkOut - more resources
Full Text Sources
VSports app下载 - Other Literature Sources
Molecular Biology Databases

