An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans
- PMID: 23509360
- PMCID: PMC3622194
- DOI: 10.4049/jimmunol.1202671
VSports注册入口 - An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans
VSports - Abstract
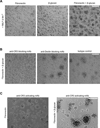
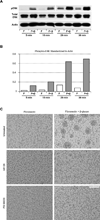
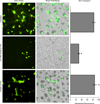
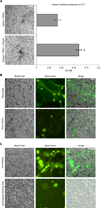
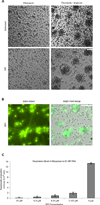
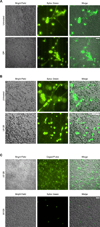
The armament of neutrophil-mediated host defense against pathogens includes the extrusion of a lattice of DNA and microbicidal enzymes known as neutrophil extracellular traps (NETs). The receptor/ligand interactions and intracellular signaling mechanisms responsible for elaborating NETs were determined for the response to Candida albicans. Because the host response of extravasated neutrophils to mycotic infections within tissues necessitates contact with extracellular matrix, this study also identified a novel and significant regulatory role for the ubiquitous matrix component fibronectin (Fn) in NET release. We report that recognition of purified fungal pathogen-associated molecular pattern β-glucan by human neutrophils causes rapid (≤ 30 min) homotypic aggregation and NET release by a mechanism that requires Fn. Alone, immobilized β-glucan induces reactive oxygen species (ROS) production but not NET release, whereas in the context of Fn, ROS production is suppressed and NETs are extruded. NET release to Fn with β-glucan is robust, accounting for 17. 2 ± 3. 4% of total DNA in the cell population. Release is dependent on β-glucan recognition by complement receptor 3 (CD11b/CD18), but not Dectin-1, or ROS. The process of NET release included filling of intracellular vesicles with nuclear material that was eventually extruded VSports手机版. We identify a role for ERK in homotypic aggregation and NET release. NET formation to C. albicans hyphae was also found to depend on β-glucan recognition by complement receptor 3, require Fn and ERK but not ROS, and result in hyphal destruction. We report a new regulatory mechanism of NETosis in which the extracellular matrix is a key component of the rapid antifungal response. .
Conflict of interest statement
Conflict-of-interest disclosure: The authors claim no competing financial interests.
"VSports注册入口" Figures



References
-
- Cheng MF, Yang YL, Yao TJ, Lin CY, Liu JS, Tang RB, Yu KW, Fan YH, Hsieh KS, Ho M, Lo HJ. Risk factors for fatal candidemia caused by Candida albicans and non-albicans candida species. BMC Infect Dis. 2005;5:22–26. - "VSports在线直播" PMC - PubMed
-
- Fraser VJ, Jones M, Dunkel J, Storfer S, Medoff G, Dunagan WC. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15:414–421. - PubMed
Publication types
- VSports app下载 - Actions
- V体育ios版 - Actions
MeSH terms
- Actions (VSports手机版)
- V体育2025版 - Actions
- "V体育安卓版" Actions
- V体育ios版 - Actions
- "VSports在线直播" Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- VSports - Actions
- "V体育安卓版" Actions
- VSports app下载 - Actions
- VSports app下载 - Actions
- VSports在线直播 - Actions
Substances
- Actions (V体育官网)
Grants and funding
"VSports" LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
VSports在线直播 - Miscellaneous

