Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics
- PMID: 23479504
- PMCID: PMC3593149
- DOI: 10.1593/neo.121950
VSports注册入口 - Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics
Abstract
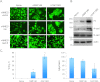
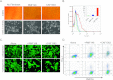
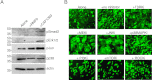
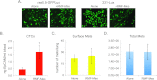
A wealth of evidence has now demonstrated that the microenvironment in which a tumorigenic cell evolves is as critical to its evolution as the genetic mutations it accrues. However, there is still relatively little known about how signals from the microenvironment contribute to the early events in the progression to malignancy. To address this question, we used a premalignant mammary model to examine how fibroblasts, and the extracellular matrix (ECM) proteins they secrete, influence progression to malignancy. Their effect on metastatic malignant cells was also assessed for comparison. We found that carcinoma-associated fibroblasts, and the distinct aligned ECM they deposit, can cause both premalignant and malignant mammary epithelial cells to assume a mesenchymal morphology that is associated with increased dissemination and metastasis, while benign reduction mammoplasty fibroblasts favor the maintenance of an epithelial morphology and constrain early dissemination, tumor growth, and metastasis. Our results suggest that normalizing the organization of the ECM could be effective in limiting systemic dissemination and tumor growth VSports手机版. .
Figures




References (VSports注册入口)
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. - PubMed
-
- Allen M, Louise Jones J. Jekyll and Hyde: the role of the micro-environment on the progression of cancer. J Pathol. 2011;223:162–176. - PubMed
-
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. - "V体育平台登录" PMC - PubMed
-
- Haviv I, Polyak K, Qiu W, Hu M, Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle. 2009;8:589–595. - PubMed
Publication types
- VSports最新版本 - Actions
- Actions (VSports在线直播)
MeSH terms
- "VSports最新版本" Actions
- V体育官网 - Actions
- V体育官网入口 - Actions
- "V体育安卓版" Actions
- "VSports" Actions
- "V体育安卓版" Actions
- Actions (VSports)
- Actions (VSports最新版本)
- Actions (V体育2025版)
- VSports注册入口 - Actions
Substances
VSports注册入口 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
