Acid-induced mechanism change and overpotential decrease in dioxygen reduction catalysis with a dinuclear copper complex (VSports app下载)
- PMID: 23442145
- PMCID: PMC3596431
- DOI: 10.1021/ja312256u
Acid-induced mechanism change and overpotential decrease in dioxygen reduction catalysis with a dinuclear copper complex (V体育安卓版)
Abstract
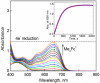
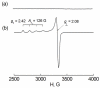
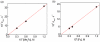
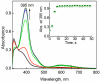
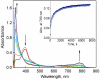
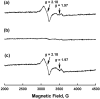
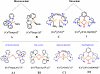
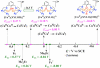
Catalytic four-electron reduction of O2 by ferrocene (Fc) and 1,1'-dimethylferrocene (Me2Fc) occurs efficiently with a dinuclear copper(II) complex [Cu(II)2(XYLO)(OH)](2+) (1), where XYLO is a m-xylene-linked bis[(2-(2-pyridyl)ethyl)amine] dinucleating ligand with copper-bridging phenolate moiety], in the presence of perchloric acid (HClO4) in acetone at 298 K. The hydroxide and phenoxo group in [Cu(II)2(XYLO)(OH)](2+) (1) undergo protonation with HClO4 to produce [Cu(II)2(XYLOH)](4+) (2) where the two copper centers become independent and the reduction potential shifts from -0. 68 V vs SCE in the absence of HClO4 to 0. 47 V; this makes possible the use of relatively weak one-electron reductants such as Fc and Me2Fc, significantly reducing the effective overpotential in the catalytic O2-reduction reaction. The mechanism of the reaction has been clarified on the basis of kinetic studies on the overall catalytic reaction as well as each step in the catalytic cycle and also by low-temperature detection of intermediates. The O2-binding to the fully reduced complex [Cu(I)2(XYLOH)](2+) (3) results in the reversible formation of the hydroperoxo complex ([Cu(II)2(XYLO)(OOH)](2+)) (4), followed by proton-coupled electron-transfer (PCET) reduction to complete the overall O2-to-2H2O catalytic conversion VSports手机版. .
Figures


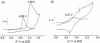


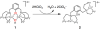
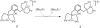
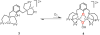
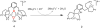

References
-
- Pereira MM, Santana M, Teixeira M. Biochim. Biophys. Acta. 2001;1505:185. - PubMed
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. Science. 1995;269:1069. - PubMed
- Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Science. 1998;280:1723. - PubMed
-
- Malmström BG. Acc. Chem. Res. 1993;26:332.
- Malmström BG. Chem. Rev. 1990;90:1247.
- Winter M, Brodd RJ. Chem. Rev. 2004;104:4245. - V体育官网 - PubMed
-
- Ferguson-Miller S, Babcock GT. Chem. Rev. 1996;96:2889. - "V体育ios版" PubMed
- Hosler JP, Ferguson-Miller S, Mills DA. Annu. Rev. Biochem. 2006;75:165. - PMC - PubMed
- Kaila VRI, Verkhovsky MI, Wikstróm M. Chem. Rev. 2010;110:7062. - PubMed
-
- Kim E, Chufán EE, Kamaraj K, Karlin KD. Chem. Rev. 2004;104:1077. - PubMed (V体育安卓版)
- Chufàn EE, Puiu SC, Karlin KD. Acc. Chem. Res. 2007;40:563. - PubMed (V体育平台登录)
- Collman JP, Boulatov R, Sunderland CJ, Fu L. Chem. Rev. 2004;104:561. - PubMed
-
- Babcock GT, Wikström M. Nature. 1992;356:301. - PubMed
V体育2025版 - Publication types
- Actions (V体育安卓版)
- VSports在线直播 - Actions
MeSH terms (V体育官网入口)
- "V体育官网入口" Actions
- Actions (VSports注册入口)
"V体育安卓版" Substances
- VSports注册入口 - Actions
- Actions (VSports手机版)
- "VSports app下载" Actions
Grants and funding
LinkOut - more resources (V体育安卓版)
"V体育2025版" Full Text Sources
Other Literature Sources
Miscellaneous

