Anti-KIT designer T cells for the treatment of gastrointestinal stromal tumor
- PMID: 23433424
- PMCID: PMC3599052
- DOI: 10.1186/1479-5876-11-46
Anti-KIT designer T cells for the treatment of gastrointestinal stromal tumor
Abstract
Background: Imatinib mesylate is an effective treatment for metastatic gastrointestinal stromal tumor (GIST). However, most patients eventually develop resistance and there are few other treatment options VSports手机版. Immunotherapy using genetically modified or designer T cells (dTc) has gained increased attention for several malignancies in recent years. The aims of this study were to develop and test novel anti-KIT dTc engineered to target GIST cells. .
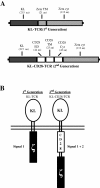
Methods: Human anti-KIT dTc were created by retroviral transduction with novel chimeric immune receptors (CIR). The gene for stem cell factor (SCF), the natural ligand for KIT, was cloned into 1st generation (SCF-CD3ζ, 1st gen) and 2nd generation (SCF-CD28-CD3ζ, 2nd gen) CIR constructs. In vitro dTc proliferation and tumoricidal capacity in the presence of KIT+ tumor cells were measured. In vivo assessment of dTc anti-tumor efficacy was performed by treating immunodeficient mice harboring subcutaneous GIST xenografts with dTc tail vein infusions. V体育安卓版.
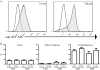
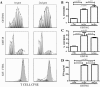
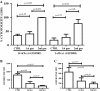
Results: We successfully produced the 1st and 2nd gen anti-KIT CIR and transduced murine and human T cells. Average transduction efficiencies for human 1st and 2nd gen dTc were 50% and 42%. When co-cultured with KIT+ tumor cells, both 1st and 2nd gen dTc proliferated and produced IFNγ. Human anti-KIT dTc were efficient at lysing GIST in vitro compared to untransduced T cells. In mice with established GIST xenografts, treatment with either 1st or 2nd gen human anti-KIT dTc led to significant reductions in tumor growth rates. V体育ios版.
Conclusions: We have constructed a novel anti-KIT CIR for production of dTc that possess specific activity against KIT+ GIST in vitro and in vivo VSports最新版本. Further studies are warranted to evaluate the therapeutic potential and safety of anti-KIT dTc. .
Figures
References
-
- Parkkila S, Lasota J, Fletcher JA, Ou WB, Kivela AJ, Nuorva K, Parkkila AK, Ollikainen J, Sly WS, Waheed A. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod Pathol. 2010;23:743–750. doi: 10.1038/modpathol.2009.189. - "V体育官网入口" DOI - PMC - PubMed
-
- Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. - DOI - PMC - PubMed
-
- Janicke RU, Engels IH, Dunkern T, Kaina B, Schulze-Osthoff K, Porter AG. Ionizing radiation but not anticancer drugs causes cell cycle arrest and failure to activate the mitochondrial death pathway in MCF-7 breast carcinoma cells. Oncogene. 2001;20:5043–5053. doi: 10.1038/sj.onc.1204659. - DOI - PubMed
"VSports" Publication types
MeSH terms
- "VSports最新版本" Actions
- Actions (V体育2025版)
- "V体育安卓版" Actions
- Actions (VSports app下载)
- Actions (V体育安卓版)
- V体育平台登录 - Actions
- Actions (V体育安卓版)
Substances
- Actions (VSports注册入口)
LinkOut - more resources
"V体育平台登录" Full Text Sources
"VSports手机版" Other Literature Sources

