IL-21 promotes the expansion of CD27+ CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells
- PMID: 23402380
- PMCID: PMC3626797
- DOI: 10.1186/1479-5876-11-37
VSports - IL-21 promotes the expansion of CD27+ CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells
"V体育ios版" Abstract
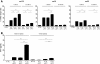
Background: Adoptive cell transfer of tumor infiltrating lymphocytes has shown clinical efficacy in the treatment of melanoma and is now also being explored in other tumor types. Generation of sufficient numbers of effector T cells requires extensive ex vivo expansion, often at the cost of T cell differentiation and potency VSports手机版. For the past 20 years, IL-2 has been the key cytokine applied in the expansion of TIL for ACT. However, the use of IL-2 has also led to collateral expansion of regulatory T cells (Tregs) and progressive T cell differentiation, factors known to limit in vivo persistence and activity of transferred TIL. The use of alternative T cell growth factors is therefore warranted. Here, we have compared the effects of IL-2, -15 and -21 cytokines on the expansion and activation of TIL from single-cell suspensions of non-small cell lung cancer, ovarian cancer and melanoma. .
Methods: We applied the K562-based artificial APC (aAPC) platform for the direct and rapid expansion of tumor infiltrating lymphocytes isolated from primary cancer specimens. These aAPC were engineered to express the Fc-γ receptor CD32 (for anti-CD3 antibody binding), the co-stimulatory molecule 4-1BBL, and to secrete either IL-2, IL-15 or IL-21 cytokine V体育安卓版. .
Results: Although IL-2 aAPC induced the greatest overall TIL expansion, IL-21 aAPC induced superior expansion of CD8+ T cells with a CD27+ CD28+ "young" phenotype and superior functional cytotoxic effector characteristics, without collateral expansion of Tregs V体育ios版. .
Conclusion: Our data rationalize the clinical application of IL-21-secreting aAPC as a standardized cell-based platform in the expansion of "young" effector TIL for ACT. VSports最新版本.
Figures



References
-
- Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. - DOI - PMC - PubMed
-
- Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, Restifo NP, Wunderlich JR, Prieto PA, Hong JJ, Langan RC, Zlott DA, Morton KE, White DE, Laurencot CM, Rosenberg SA. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. - DOI - PMC - PubMed
-
- Itzhaki O, Hovav E, Ziporen Y, Levy D, Kubi A, Zikich D, Hershkovitz L, Treves AJ, Shalmon B, Zippel D, Markel G, Shapira-Frommer R, Schachter J, Besser MJ. Establishment and large-scale expansion of minimally cultured "young" tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–220. - PubMed
Publication types
MeSH terms
- V体育2025版 - Actions
- Actions (V体育2025版)
- VSports app下载 - Actions
- VSports最新版本 - Actions
- "VSports app下载" Actions
- Actions (V体育安卓版)
- "VSports" Actions
- Actions (VSports app下载)
- V体育官网 - Actions
Substances (VSports手机版)
- Actions (V体育平台登录)
- "VSports在线直播" Actions
- V体育官网 - Actions
"V体育平台登录" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育ios版" Research Materials
Miscellaneous

