"V体育ios版" The tumor suppressor Mst1 promotes changes in the cellular redox state by phosphorylation and inactivation of peroxiredoxin-1 protein
- PMID: 23386615
- PMCID: PMC3605693
- DOI: 10.1074/jbc.M112.414524
"VSports最新版本" The tumor suppressor Mst1 promotes changes in the cellular redox state by phosphorylation and inactivation of peroxiredoxin-1 protein
Abstract (V体育安卓版)
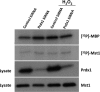
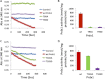
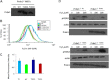
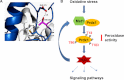
The serine/threonine protein kinases Mst1 and Mst2 can be activated by cellular stressors including hydrogen peroxide. Using two independent protein interaction screens, we show that these kinases associate, in an oxidation-dependent manner, with Prdx1, an enzyme that regulates the cellular redox state by reducing hydrogen peroxide to water and oxygen. Mst1 inactivates Prdx1 by phosphorylating it at Thr-90 and Thr-183, leading to accumulation of hydrogen peroxide in cells. These results suggest that hydrogen peroxide-stimulated Mst1 activates a positive feedback loop to sustain an oxidizing cellular state. VSports手机版.
Figures



VSports注册入口 - References
-
- Song H., Mak K. K., Topol L., Yun K., Hu J., Garrett L., Chen Y., Park O., Chang J., Simpson R. M., Wang C. Y., Gao B., Jiang J., Yang Y. (2010) Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. U.S.A. 107, 1431–1436 - PMC (VSports) - PubMed
-
- Zhou D., Conrad C., Xia F., Park J. S., Payer B., Yin Y., Lauwers G. Y., Thasler W., Lee J. T., Avruch J., Bardeesy N. (2009) Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 - PMC - PubMed
-
- Zeng Q., Hong W. (2008) The emerging role of the Hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13, 188–192 - PubMed
Publication types
- V体育安卓版 - Actions
- V体育安卓版 - Actions
MeSH terms
- "V体育ios版" Actions
- "VSports手机版" Actions
- "VSports app下载" Actions
- VSports最新版本 - Actions
- Actions (VSports手机版)
- VSports注册入口 - Actions
- Actions (V体育2025版)
- "V体育官网" Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- VSports在线直播 - Actions
Substances
- V体育平台登录 - Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
"V体育官网" Grants and funding
V体育2025版 - LinkOut - more resources
"VSports" Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

