VSports在线直播 - Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation?
- PMID: 23383243
- PMCID: PMC3561332
- DOI: 10.1371/journal.pone.0055612
Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation?
Erratum in
- PLoS One. 2013;8(10). doi:10.1371/annotation/0faf4c12-4fd4-474c-b5a4-16e49370aff3
"VSports app下载" Abstract
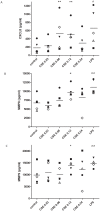
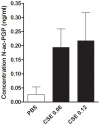
Background: Cigarette smoking induces inflammatory responses in all smokers and is the major risk factor for lung disease such as chronic obstructive pulmonary disease (COPD). In this progressive disease, chronic inflammation in the lung contributes to lung tissue destruction leading to the formation of chemotactic collagen fragments such as N-acetylated Proline-Glycine-Proline (N-ac-PGP). The generation of this tripeptide is mediated by a multistep pathway involving matrix metalloproteases (MMPs) 8 and 9 and prolyl endopeptidase (PE). Here we investigated whether cigarette smoke extract (CSE) stimulates human PMNs to breakdown whole matrix collagen leading to the generation of the chemotactic collagen fragment N-ac-PGP. VSports手机版.
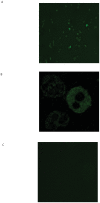
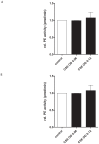
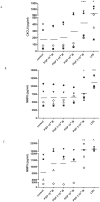
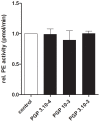
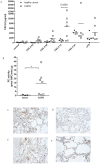
Methodology/principal findings: Incubating PMNs with CSE led to the release of chemo-attractant CXCL8 and proteases MMP8 and MMP9. PMNs constitutively expressed PE activity as well as PE protein. Incubating CSE-primed PMNs with collagen resulted in collagen breakdown and in N-ac-PGP generation. Incubation of PMNs with the tripeptide N-ac-PGP resulted in the release of CXCL8, MMP8 and MMP9 V体育安卓版. Moreover, we tested whether PMNs from COPD patients are different from PMNs from healthy donors. Here we show that the intracellular basal PE activity of PMNs from COPD patients increased 25-fold compared to PMNs from healthy donors. Immunohistological staining of human lung tissue for PE showed that besides neutrophils, macrophages and epithelial cells express PE. .
Conclusions: This study indicates that neutrophils activated by cigarette smoke extract can breakdown collagen into N-ac-PGP and that this collagen fragment itself can activate neutrophils, which may lead in vivo to a self-propagating cycle of neutrophil infiltration, chronic inflammation and lung emphysema. MMP-, PE- or PGP-inhibitors can serve as an attractive therapeutic target and may open new avenues towards effective treatment of COPD. V体育ios版.
Conflict of interest statement (VSports最新版本)
Competing Interests: JG is employed by a commercial company, Danone Research. However, this does not alter the authors’ adherence to all the PLOS ONE policies on sharing data and materials VSports最新版本.
Figures

References (VSports在线直播)
-
- Barnes PJ (2004) Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 56: 515–548. - PubMed
-
- Stockley RA, Mannino D, Barnes PJ (2009) Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 524–526. - PubMed
-
- Barnes PJ (2010) New therapies for chronic obstructive pulmonary disease. Med Princ Pract 19: 330–338. - PubMed
-
- Quint JK, Wedzicha JA (2007) The neutrophil in chronic obstructive pulmonary disease. J Allergy Clin Immunol 119: 1065–1071. - PubMed
-
- Mannino DM, Buist AS (2007) Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370: 765–773. - PubMed
Publication types
- "VSports app下载" Actions
MeSH terms
- "V体育安卓版" Actions
- Actions (V体育官网入口)
- Actions (V体育ios版)
- "VSports app下载" Actions
- "V体育官网" Actions
- VSports - Actions
- Actions (VSports app下载)
- Actions (VSports手机版)
- Actions (V体育官网)
- "V体育ios版" Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- "V体育ios版" Actions
- Actions (V体育2025版)
- "VSports" Actions
- "VSports最新版本" Actions
- "VSports" Actions
- Actions (V体育官网入口)
Substances
- VSports注册入口 - Actions
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"V体育ios版" Miscellaneous

