Redox modulation of HMGB1-related signaling
- PMID: 23373897
- PMCID: PMC3928832 (VSports在线直播)
- DOI: 10.1089/ars.2013.5179
Redox modulation of HMGB1-related signaling
Abstract
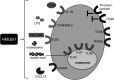
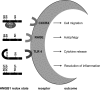
Significance: In the cells' nuclei, high-mobility group box protein 1 (HMGB1) is a nonhistone chromatin-binding protein involved in the regulation of transcription. Extracellularly, HMGB1 acts as a danger molecule with properties of a proinflammatory cytokine. It can be actively secreted from myeloid cells or passively leak from any type of injured, necrotic cell. Increased serum levels of active HMGB1 are often found in pathogenic inflammatory conditions and correlate with worse prognoses in cancer, sepsis, and autoimmunity VSports手机版. By damaging cells, superoxide and peroxynitrite promote leakage of HMGB1. .
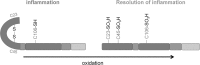
Recent advances: The activity of HMGB1 strongly depends on its redox state: Inflammatory-active HMGB1 requires an intramolecular disulfide bond (Cys23 and Cys45) and a reduced Cys106. Oxidation of the latter blocks its stimulatory activity and promotes immune tolerance. V体育安卓版.
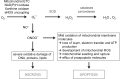
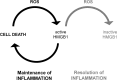
Critical issues: Reactive oxygen and nitrogen species create an oxidative environment and can be detoxified by superoxide dismutase (SOD), catalase, and peroxidases. Modifications of the oxidative environment influence HMGB1 activity V体育ios版. .
Future directions: In this review, we hypothesize that manipulations of an oxidative environment by SOD mimics or by hydrogen sulfide are prone to decrease tissue damage. Both the concomitant decreased HMGB1 release and its redox chemical modifications ameliorate inflammation and tissue damage VSports最新版本. .
Figures


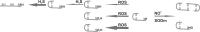
References
-
- Abdulahad DA, Westra J, Limburg PC, Kallenberg CG, and Bijl M. HMGB1 in systemic lupus Erythematosus: its role in cutaneous lesions development. Autoimmun Rev 9: 661–665, 2010 - PubMed
-
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, and Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117: 3216–3226, 2008 - PubMed
-
- Bianchi ME. HMGB1 loves company. J Leukoc Biol 86: 573–576, 2009 - PubMed
-
- Bianchi ME. and Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev 15: 496–506, 2005 - PubMed
Publication types
- "VSports在线直播" Actions
MeSH terms
- Actions (V体育平台登录)
- "V体育官网" Actions
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
