VSports最新版本 - A role for Ly108 in the induction of promyelocytic zinc finger transcription factor in developing thymocytes
- PMID: 23355739
- PMCID: PMC3578000
- DOI: 10.4049/jimmunol.1202145
"VSports在线直播" A role for Ly108 in the induction of promyelocytic zinc finger transcription factor in developing thymocytes
Abstract
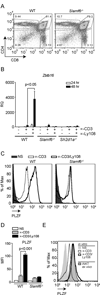
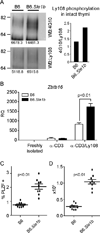
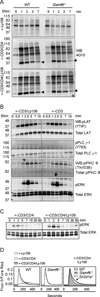
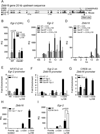
The promyelocytic zinc finger transcription factor (PLZF) is required for the development of activated phenotypes in NKT and other innate T lymphocytes. Although strong TCR stimulation has been implicated in the induction of PLZF, the factors regulating PLZF expression are incompletely understood. We show in this study that costimulation of preselection double-positive thymocytes through the signaling lymphocyte activation molecule family receptor Ly108 markedly enhanced PLZF expression compared with that induced by TCR stimulation alone. Costimulation with Ly108 increased expression of early growth response protein (Egr)-2 and binding of Egr-2 to the promoter of Zbtb16, which encodes PLZF, and resulted in PLZF levels similar to those seen in NKT cells. In contrast, costimulation with anti-CD28 failed to enhance Egr-2 binding and Zbtb16 expression. Moreover, mice lacking Ly108 showed decreased numbers of PLZF-expressing CD4(+) T cells. Together, these results support a potential role for Ly108 in the induction of PLZF. VSports手机版.
Figures



"VSports app下载" References
-
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci U S A. 2009;106:12453–12458. - PMC - PubMed
-
- Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant'Angelo DB. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. Journal of immunology. 2010;184:6746–6755. - PubMed
Publication types
- Actions (V体育官网入口)
- V体育官网 - Actions
MeSH terms
- "VSports最新版本" Actions
- V体育平台登录 - Actions
- "V体育2025版" Actions
- VSports手机版 - Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- VSports app下载 - Actions
- Actions (V体育ios版)
- VSports最新版本 - Actions
- VSports最新版本 - Actions
- Actions (V体育安卓版)
"V体育安卓版" Substances
- V体育官网入口 - Actions
- "V体育2025版" Actions
- VSports - Actions
- "V体育官网" Actions
- "V体育平台登录" Actions
Grants and funding
LinkOut - more resources (V体育平台登录)
"VSports app下载" Full Text Sources
"V体育官网入口" Other Literature Sources
Molecular Biology Databases
Research Materials

