COX19 mediates the transduction of a mitochondrial redox signal from SCO1 that regulates ATP7A-mediated cellular copper efflux (VSports注册入口)
- PMID: 23345593
- PMCID: "VSports在线直播" PMC3596241
- DOI: 10.1091/mbc.E12-09-0705 (VSports)
COX19 mediates the transduction of a mitochondrial redox signal from SCO1 that regulates ATP7A-mediated cellular copper efflux
Abstract
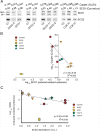
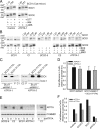
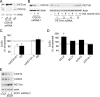
SCO1 and SCO2 are metallochaperones whose principal function is to add two copper ions to the catalytic core of cytochrome c oxidase (COX). However, affected tissues of SCO1 and SCO2 patients exhibit a combined deficiency in COX activity and total copper content, suggesting additional roles for these proteins in the regulation of cellular copper homeostasis. Here we show that both the redox state of the copper-binding cysteines of SCO1 and the abundance of SCO2 correlate with cellular copper content and that these relationships are perturbed by mutations in SCO1 or SCO2, producing a state of apparent copper overload. The copper deficiency in SCO patient fibroblasts is rescued by knockdown of ATP7A, a trans-Golgi, copper-transporting ATPase that traffics to the plasma membrane during copper overload to promote efflux. To investigate how a signal from SCO1 could be relayed to ATP7A, we examined the abundance and subcellular distribution of several soluble COX assembly factors VSports手机版. We found that COX19 partitions between mitochondria and the cytosol in a copper-dependent manner and that its knockdown partially rescues the copper deficiency in patient cells. These results demonstrate that COX19 is necessary for the transduction of a SCO1-dependent mitochondrial redox signal that regulates ATP7A-mediated cellular copper efflux. .
Figures


References
-
- Andruzzi L, Nakano M, Nilges MJ, Blackburn NJ. Spectroscopic studies of metal binding and metal selectivity in Bacillus subtilis BSco, a homologue of the yeast mitochondrial protein Sco1p. J Am Chem Soc. 2005;127:16548–16558. - "VSports app下载" PubMed
-
- Antonicka H, Leary SC, Guercin GH, Agar JN, Horvath R, Kennaway NG, Harding CO, Jaksch M, Shoubridge EA. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum Mol Genet. 2003a;12:2693–2702. - PubMed
-
- Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am J Hum Genet. 2003b;72:101–114. - V体育2025版 - PMC - PubMed
-
- Balatri E, Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. Solution structure of Sco1: a thioredoxin-like protein Involved in cytochrome c oxidase assembly. Structure. 2003;11:1431–1443. - V体育平台登录 - PubMed
-
- Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, Martinelli M, Palumaa P, Wang S. A hint for the function of human Sco1 from different structures. Proc Natl Acad Sci USA. 2006a;103:8595–8600. - PMC (VSports) - PubMed
Publication types
MeSH terms
- Actions (V体育ios版)
- Actions (V体育官网入口)
- "VSports app下载" Actions
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- V体育官网 - Actions
- Actions (VSports在线直播)
- V体育官网 - Actions
"V体育官网" Substances
- "VSports app下载" Actions
- VSports手机版 - Actions
- "V体育ios版" Actions
- "V体育官网" Actions
- V体育官网入口 - Actions
- "VSports app下载" Actions
Grants and funding
VSports在线直播 - LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports在线直播" Molecular Biology Databases

