Role of host xanthine oxidase in infection due to enteropathogenic and Shiga-toxigenic Escherichia coli (VSports手机版)
- PMID: 23340314
- PMCID: PMC3639607 (V体育官网)
- DOI: "VSports注册入口" 10.1128/IAI.01124-12
Role of host xanthine oxidase in infection due to enteropathogenic and Shiga-toxigenic Escherichia coli
Abstract (V体育官网入口)
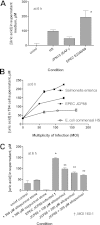
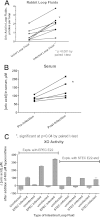
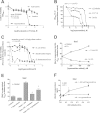
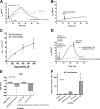
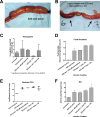
Xanthine oxidase (XO), also known as xanthine oxidoreductase, has long been considered an important host defense molecule in the intestine and in breastfed infants. Here, we present evidence that XO is released from and active in intestinal tissues and fluids in response to infection with enteropathogenic Escherichia coli (EPEC) and Shiga-toxigenic E. coli (STEC), also known as enterohemorrhagic E VSports手机版. coli (EHEC). XO is released into intestinal fluids in EPEC and STEC infection in a rabbit animal model. XO activity results in the generation of surprisingly high concentrations of uric acid in both cultured cell and animal models of infection. Hydrogen peroxide (H(2)O(2)) generated by XO activity triggered a chloride secretory response in intestinal cell monolayers within minutes but decreased transepithelial electrical resistance at 6 to 22 h. H(2)O(2) generated by XO activity was effective at killing laboratory strains of E. coli, commensal microbiotas, and anaerobes, but wild-type EPEC and STEC strains were 100 to 1,000 times more resistant to killing or growth inhibition by this pathway. Instead of killing pathogenic bacteria, physiologic concentrations of XO increased virulence by inducing the production of Shiga toxins from STEC strains. In vivo, exogenous XO plus the substrate hypoxanthine did not protect and instead worsened the outcome of STEC infection in the rabbit ligated intestinal loop model of infection. XO released during EPEC and STEC infection may serve as a virulence-inducing signal to the pathogen and not solely as a protective host defense. .
Figures
Comment in
-
Role of host xanthine oxidase in infection due to enteropathogenic and Shiga-toxigenic Escherichia coli.Gut Microbes. 2013 Sep-Oct;4(5):388-91. doi: 10.4161/gmic.25584. Epub 2013 Jun 28. Gut Microbes. 2013. PMID: 23811846 Free PMC article.
References (V体育ios版)
-
- Stevens C, Millar T, Clinch J, Kanczler J, Bodamyali T, Blake D. 2000. Antibacterial properties of xanthine oxidase in human milk. Lancet 356:829–830 - PubMed
-
- Los JM, Los M, Wêgrzyn A, Wêgrzyn G. 2010. Hydrogen peroxide-mediated induction of the Shiga toxin-converting lambdoid prophage ST2-8624 in Escherichia coli O157:H7. FEMS Immunol. Med. Microbiol. 58(3):322–329 - PubMed
Publication types
- "VSports注册入口" Actions
MeSH terms
- Actions (V体育安卓版)
- Actions (V体育2025版)
- Actions (VSports注册入口)
- "VSports" Actions
- "V体育2025版" Actions
- V体育官网 - Actions
- "V体育官网" Actions
- VSports注册入口 - Actions
- "VSports注册入口" Actions
- "V体育官网" Actions
Substances
Grants and funding
"V体育官网" LinkOut - more resources
Full Text Sources
"V体育2025版" Other Literature Sources
VSports注册入口 - Medical
V体育ios版 - Molecular Biology Databases

