VSports注册入口 - The function of hematopoietic stem cells is altered by both genetic and inflammatory factors in lupus mice
- PMID: 23315165
- PMCID: PMC3596961 (VSports)
- DOI: 10.1182/blood-2012-05-433755
The function of hematopoietic stem cells is altered by both genetic and inflammatory factors in lupus mice (VSports手机版)
Abstract
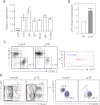
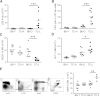
Hematopoietic stem cells (HSCs) are protected in a metabolically dormant state within the bone marrow stem cell niche. Inflammation has been shown to disrupt HSC dormancy and cause multiple functional changes. Here, we investigated whether HSC functions were altered in systemic lupus erythematosus (SLE)-prone mice and whether this contributed to clinical manifestations of SLE. We found that HSCs were significantly expanded in lupus mice. The increase in HSC cellularity was caused by both genetic lupus risk factors and inflammatory cytokines in lupus mice. In addition, the inflammatory conditions of lupus led to HSC mobilization and lineage-biased hematopoiesis. Strikingly, these functionally altered HSCs possessed robust self-renewal capacity and exhibited repopulating advantages over wild-type HSCs. A single-nucleotide polymorphism in the cdkn2c gene encoding p18(INK4c) within a SLE susceptibility locus was found to account for reduced p18(INK4c) expression and the increase in HSC self-renewal capacity in lupus mice. Lupus HSCs with enhanced self-renewal capacity and resistance to stress may compete out transplanted healthy HSCs, thereby leading to relapses after HSC transplantation. VSports手机版.
"V体育2025版" Figures




References
-
- Cheshier SH, Morrison SJ, Liao X, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96(6):3120–3125. - VSports最新版本 - PMC - PubMed
-
- Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. - PubMed
-
- Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–1808. - PubMed
-
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453(7193):306–313. - V体育ios版 - PubMed
-
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. - PubMed
Publication types
- "VSports注册入口" Actions
MeSH terms
- Actions (V体育安卓版)
- "VSports手机版" Actions
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- "V体育ios版" Actions
- Actions (V体育安卓版)
- "V体育ios版" Actions
- V体育官网 - Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
Substances
- Actions (V体育平台登录)
V体育安卓版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

