A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition (VSports app下载)
- PMID: 23288408
- PMCID: PMC3606893
- DOI: 10.1158/2159-8290.CD-12-0470
A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition
Abstract
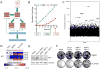
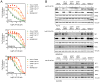
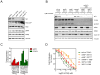
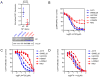
RAF inhibitors such as vemurafenib and dabrafenib block BRAF-mediated cell proliferation and achieve meaningful clinical benefit in the vast majority of patients with BRAF(V600E)-mutant melanoma. However, some patients do not respond to this regimen, and nearly all progress to therapeutic resistance. We used a pooled RNA interference screen targeting more than 16,500 genes to discover loss-of-function events that could drive resistance to RAF inhibition. The highest ranking gene was NF1, which encodes neurofibromin, a tumor suppressor that inhibits RAS activity. NF1 loss mediates resistance to RAF and mitogen-activated protein kinase (MAPK) kinase kinase (MEK) inhibitors through sustained MAPK pathway activation. However, cells lacking NF1 retained sensitivity to the irreversible RAF inhibitor AZ628 and an ERK inhibitor. NF1 mutations were observed in BRAF-mutant tumor cells that are intrinsically resistant to RAF inhibition and in melanoma tumors obtained from patients exhibiting resistance to vemurafenib, thus showing the clinical potential for NF1-driven resistance to RAF/MEK-targeted therapies VSports手机版. .
Conflict of interest statement
Conflicts of interest: L. A. G. is a consultant for Foundation Medicine, Novartis, and Millennium/Takeda, and an equity holder in Foundation Medicine V体育安卓版.
Figures (VSports最新版本)


Comment in (VSports在线直播)
-
An unholy alliance: cooperation between BRAF and NF1 in melanoma development and BRAF inhibitor resistance. (VSports app下载)Cancer Discov. 2013 Mar;3(3):260-3. doi: 10.1158/2159-8290.CD-13-0017. Cancer Discov. 2013. PMID: 23475878 Free PMC article.
References
-
- Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18:73–9. - PubMed
-
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–14. - "VSports在线直播" PubMed
-
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. - PubMed
Publication types (VSports)
- VSports手机版 - Actions
MeSH terms
- "V体育官网" Actions
- "VSports最新版本" Actions
- Actions (VSports)
- "VSports注册入口" Actions
- V体育官网 - Actions
- Actions (VSports最新版本)
- V体育官网 - Actions
"VSports最新版本" Substances
- Actions (V体育安卓版)
- Actions (VSports)
Grants and funding
LinkOut - more resources
"VSports" Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
"V体育平台登录" Research Materials
Miscellaneous (V体育平台登录)

