Direct interaction of Bax and Bak proteins with Bcl-2 homology domain 3 (BH3)-only proteins in living cells revealed by fluorescence complementation (VSports app下载)
- PMID: 23283967
- PMCID: PMC3576097
- DOI: 10.1074/jbc.M112.422204
Direct interaction of Bax and Bak proteins with Bcl-2 homology domain 3 (BH3)-only proteins in living cells revealed by fluorescence complementation (V体育2025版)
Abstract (VSports注册入口)
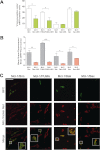
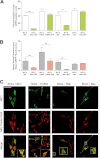
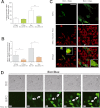
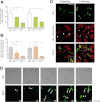
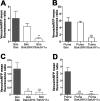
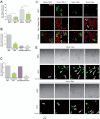
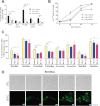
The key event in the mitochondrial pathway of apoptosis is the activation of Bax and Bak by BH3-only proteins through a molecular mechanism that is still a matter of debate. Here we studied interactions among anti- and proapoptotic proteins of the Bcl-2 family in living cells by using bimolecular fluorescence complementation analysis. Our results indicate that the antiapoptotic proteins Mcl-1 and Bcl-x(L) bind preferably to the BH3-only proteins Bim, PUMA, and Noxa but can also bind to Bak and Bax. We also found a direct interaction between Bim, PUMA, or Noxa with either Bax or Bak during apoptosis induction VSports手机版. In HeLa cells, interaction of Bim with Bax occurs in cytosol, and then Bim-Bax complexes translocate to mitochondria. Complexes of either PUMA or Noxa with Bax or Bak were always detected at mitochondria. Overexpression of Bcl-x(L) or Mcl-1 delayed Bim/Bax translocation to mitochondria. These results reveal the ability of main BH3-only proteins to directly activate Bax and Bak in living cells and suggest that a complex network of interactions regulate the function of Bcl-2 family members during apoptosis. .
Figures

V体育安卓版 - References
-
- Youle R. J., Strasser A. (2008) The BCL-2 protein family. Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 - PubMed
-
- López-Royuela N., Pérez-Galán P., Galán-Malo P., Yuste V. J., Anel A., Susín S. A., Naval J., Marzo I. (2010) Different contribution of BH3-only proteins and caspases to doxorubicin-induced apoptosis in p53-deficient leukemia cells. Biochem. Pharmacol. 79, 1746–1758 - PubMed
-
- Buckbinder L., Talbott R., Velasco-Miguel S., Takenaka I., Faha B., Seizinger B. R., Kley N. (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377, 646–649 - PubMed
Publication types (VSports手机版)
"V体育官网入口" MeSH terms
- "VSports app下载" Actions
- "VSports注册入口" Actions
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- V体育2025版 - Actions
- Actions (VSports在线直播)
- Actions (VSports app下载)
- Actions (VSports app下载)
- "VSports app下载" Actions
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- VSports在线直播 - Actions
Substances
- VSports注册入口 - Actions
- "V体育ios版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

