Neddylation pathway regulates T-cell function by targeting an adaptor protein Shc and a protein kinase Erk signaling
- PMID: 23267066
- PMCID: V体育官网入口 - PMC3545825
- DOI: V体育安卓版 - 10.1073/pnas.1213819110
Neddylation pathway regulates T-cell function by targeting an adaptor protein Shc and a protein kinase Erk signaling
Abstract
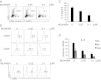
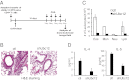
NEDD8 (neural precursor cell expressed, developmentally down-regulated 8) is a ubiquitin-like molecule whose action on modifying protein substrates is critical in various cellular functions but whose importance in the immune system is not well understood. Here we investigated the role of protein neddylation in regulating T-cell function using an in vivo knockdown technique VSports手机版. We found that reduced expression of Ubc12 in CD4(+) T cells led to impaired T-cell receptor/CD28-induced proliferation and cytokine production both in vitro and in vivo, accompanied by reduced Erk activation. These findings were recapitulated by treatment with MLN4924, an inhibitor of NEDD8-activating enzyme. Furthermore, Shc, an adaptor molecule between antigen receptors and the Ras/Erk pathway, was identified as a target for neddylation. Importantly, mice adoptively transferred with Ubc12 knockdown CD4(+) T cells showed markedly ameliorated allergic responses. This study thus identifies an important role for protein neddylation in T-cell function, which may serve as a therapeutic target for inflammatory diseases. .
V体育2025版 - Conflict of interest statement
The authors declare no conflict of interest.
Figures


V体育官网入口 - References
-
- Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: A reversible process of modification. Nat Rev Immunol. 2005;5(12):941–952. - "V体育官网入口" PMC - PubMed
-
- Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. - PubMed
-
- Harper JW, Schulman BA. Structural complexity in ubiquitin recognition. Cell. 2006;124(6):1133–1136. - V体育2025版 - PubMed
Publication types
- Actions (V体育官网)
MeSH terms (VSports手机版)
- Actions (VSports在线直播)
- Actions (V体育官网)
- "V体育2025版" Actions
- Actions (V体育ios版)
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- V体育官网 - Actions
- "VSports手机版" Actions
Substances
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- V体育平台登录 - Actions
- "V体育官网入口" Actions
- Actions (V体育2025版)
- "VSports最新版本" Actions
"V体育2025版" Grants and funding
LinkOut - more resources
"V体育ios版" Full Text Sources
Molecular Biology Databases
Research Materials
"VSports最新版本" Miscellaneous

