TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation
- PMID: 23021219
- PMCID: PMC3582364
- DOI: VSports手机版 - 10.1016/j.cell.2012.07.036
V体育安卓版 - TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation
Abstract
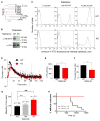
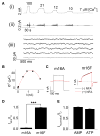
Collapse of membrane lipid asymmetry is a hallmark of blood coagulation VSports手机版. TMEM16F of the TMEM16 family that includes TMEM16A/B Ca(2+)-activated Cl(-) channels (CaCCs) is linked to Scott syndrome with deficient Ca(2+)-dependent lipid scrambling. We generated TMEM16F knockout mice that exhibit bleeding defects and protection in an arterial thrombosis model associated with platelet deficiency in Ca(2+)-dependent phosphatidylserine exposure and procoagulant activity and lack a Ca(2+)-activated cation current in the platelet precursor megakaryocytes. Heterologous expression of TMEM16F generates a small-conductance Ca(2+)-activated nonselective cation (SCAN) current with subpicosiemens single-channel conductance rather than a CaCC. TMEM16F-SCAN channels permeate both monovalent and divalent cations, including Ca(2+), and exhibit synergistic gating by Ca(2+) and voltage. We further pinpointed a residue in the putative pore region important for the cation versus anion selectivity of TMEM16F-SCAN and TMEM16A-CaCC channels. This study thus identifies a Ca(2+)-activated channel permeable to Ca(2+) and critical for Ca(2+)-dependent scramblase activity during blood coagulation. PAPERFLICK: .
Copyright © 2012 Elsevier Inc. All rights reserved V体育安卓版. .
Figures (V体育平台登录)





References
-
- Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584:2724–2730. - PubMed
-
- Bevers EM, Comfurius P, Zwaal RF. Changes in membrane phospholipid distribution during platelet activation. Biochim Biophys Acta. 1983;736:57–66. - "VSports在线直播" PubMed
-
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. - "V体育官网入口" PubMed
Publication types
- V体育平台登录 - Actions
MeSH terms
- VSports手机版 - Actions
- Actions (V体育官网入口)
- VSports - Actions
- "V体育官网" Actions
- "V体育官网入口" Actions
- "VSports app下载" Actions
- "VSports" Actions
- V体育官网 - Actions
- Actions (V体育官网入口)
- V体育2025版 - Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
V体育官网 - Substances
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports注册入口" Other Literature Sources
Molecular Biology Databases
"V体育ios版" Miscellaneous

