Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis
- PMID: 23006325
- PMCID: PMC3461916
- DOI: 10.1172/JCI63352
Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis
Abstract
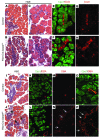
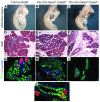
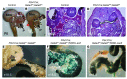
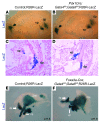
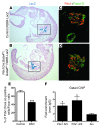
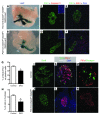
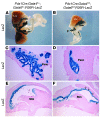
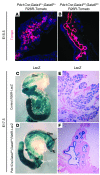
Pancreatic agenesis is a human disorder caused by defects in pancreas development. To date, only a few genes have been linked to pancreatic agenesis in humans, with mutations in pancreatic and duodenal homeobox 1 (PDX1) and pancreas-specific transcription factor 1a (PTF1A) reported in only 5 families with described cases. Recently, mutations in GATA6 have been identified in a large percentage of human cases, and a GATA4 mutant allele has been implicated in a single case. In the mouse, Gata4 and Gata6 are expressed in several endoderm-derived tissues, including the pancreas. To analyze the functions of GATA4 and/or GATA6 during mouse pancreatic development, we generated pancreas-specific deletions of Gata4 and Gata6. Surprisingly, loss of either Gata4 or Gata6 in the pancreas resulted in only mild pancreatic defects, which resolved postnatally. However, simultaneous deletion of both Gata4 and Gata6 in the pancreas caused severe pancreatic agenesis due to disruption of pancreatic progenitor cell proliferation, defects in branching morphogenesis, and a subsequent failure to induce the differentiation of progenitor cells expressing carboxypeptidase A1 (CPA1) and neurogenin 3 (NEUROG3). These studies address the conserved and nonconserved mechanisms underlying GATA4 and GATA6 function during pancreas development and provide a new mouse model to characterize the underlying developmental defects associated with pancreatic agenesis. VSports手机版.
Figures
Comment in
-
GATA believe it: new essential regulators of pancreas development.J Clin Invest. 2012 Oct;122(10):3469-71. doi: 10.1172/JCI65751. Epub 2012 Sep 24. J Clin Invest. 2012. PMID: 23006323 Free PMC article.
References (VSports在线直播)
-
- Pictet R, Rutter WJ. Handbook of Physiology, Section 7: Endocrinology. 1972. Development of the embryonic endocrine pancreas. In: Steiner DF, Freinkel N, eds. pp. 25–66. Vol. 1. Washington, DC, USA: American Physiological Society;
VSports手机版 - Publication types
MeSH terms
- Actions (VSports注册入口)
- VSports最新版本 - Actions
- "VSports在线直播" Actions
- "VSports在线直播" Actions
- Actions (V体育官网)
- Actions (VSports注册入口)
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- V体育官网入口 - Actions
- Actions (V体育官网)
- "VSports" Actions
- "V体育官网入口" Actions
- V体育平台登录 - Actions
- Actions (VSports在线直播)
- "V体育官网" Actions
- "VSports app下载" Actions
Substances
- "VSports" Actions
- "VSports最新版本" Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
Grants and funding
- DK089523/DK/NIDDK NIH HHS/United States
- R01 DK061220/DK/NIDDK NIH HHS/United States
- HL094857/HL/NHLBI NIH HHS/United States
- "V体育ios版" U01 DK089523/DK/NIDDK NIH HHS/United States
- T32 GM007088/GM/NIGMS NIH HHS/United States
- "VSports注册入口" R01 DK087711/DK/NIDDK NIH HHS/United States
- DK087711/DK/NIDDK NIH HHS/United States
- R01 DK087873/DK/NIDDK NIH HHS/United States
- "V体育平台登录" HG006398/HG/NHGRI NIH HHS/United States
- U01 HG006398/HG/NHGRI NIH HHS/United States (V体育官网)
- R01 DK055743/DK/NIDDK NIH HHS/United States
- P30 DK63608/DK/NIDDK NIH HHS/United States
- "VSports在线直播" DK61220/DK/NIDDK NIH HHS/United States
- DK55743/DK/NIDDK NIH HHS/United States
- "V体育ios版" R01 HL094857/HL/NHLBI NIH HHS/United States
- P30 DK063608/DK/NIDDK NIH HHS/United States
"V体育ios版" LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

