Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells
- PMID: 22969769
- PMCID: PMC3432456
- DOI: 10.3389/fimmu.2012.00277
Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells
Abstract (V体育平台登录)
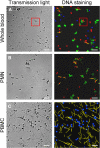
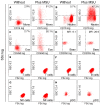
Neutrophil extracellular traps (NETs) are fibers of extracellular DNA released from neutrophils due to overwhelming phagocytic stimuli. The function of NETs is to trap and kill microbes to avoid spreading of potential pathogens. NETs are formed after encounter with various gram-positive and -negative bacteria but also in response to mediators causing sterile inflammation like interleukin-8 (IL-8), tumor necrosis factor (TNF), and phorbol myristate acetate (PMA). Here we show the formation of NETs (NETting) in response to monosodium urate (MSU) crystals as further model for sterile inflammation. We identified monocytes, neutrophils, and eosinophils as MSU phagocytosing cells. Basophils did not take up the crystals, instead they upregulated their activation marker CD203c after contact with MSU. Nevertheless, MSU crystals induced extracellular trap formation also in basophils, like in eosinophils and neutrophils, which phagocytose the crystals. In contrast, monocytes do not form NETs despite uptake of the MSU crystals. In contrast to the canonical stimuli like bacteria and PMA, MSU-induced NETosis was not abrogated by plasma VSports手机版. Our data show that MSU crystals induce extracellular DNA trap formation in all three granulocytes lineages (NETs, EETs, and BETs) but not in monocytes, and DNA externalization does not necessitate the uptake of the crystals. .
Keywords: MSU; NETs; PMA; bacteria; granulocytes; inflammation; neutrophil extracellular traps. V体育安卓版.
Figures



References
-
- Borregaard N., Cowland J. B. (1997). Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89, 3503–3521 - PubMed
-
- Burnett D., Chamba A., Stockley R. A., Murphy T. F., Hill S. L. (1993). Effects of recombinant GM-CSF and IgA opsonisation on neutrophil phagocytosis of latex beads coated with P6 outer membrane protein from Haemophilus influenzae. Thorax 48, 638–642 - PMC (VSports注册入口) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

