Manipulating the mediator: modulation of the alternative complement pathway C3 convertase in health, disease and therapy (VSports)
- PMID: 22964231
- PMCID: PMC3521040 (V体育ios版)
- DOI: 10.1016/j.imbio.2012.07.016
Manipulating the mediator: modulation of the alternative complement pathway C3 convertase in health, disease and therapy
Abstract (VSports app下载)
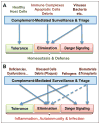
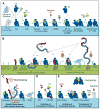
The complement network is increasingly recognized as an important triage system that is able to differentiate between healthy host cells, microbial intruders, cellular debris and immune complexes, and tailor its actions accordingly. At the center of this triage mechanism is the alternative pathway C3 convertase (C3bBb), a potent enzymatic protein complex capable of rapidly converting the inert yet abundant component C3 into powerful effector fragments (C3a and C3b), thereby amplifying the initial response on unprotected surfaces and inducing a variety of effector functions. A fascinating molecular mechanism of convertase assembly and intrinsic regulation, as well as the interplay with a panel of cell surface-bound and soluble inhibitors are essential for directing complement attack to intruders and protecting healthy host cells. While efficiently keeping immune surveillance and homeostasis on track, the reliance on an intricate cascade of interaction and conversion steps also renders the C3 convertase vulnerable to derail. On the one hand, tissue damage, accumulation of debris, or polymorphisms in complement genes may unfavorably shift the balance between activation and regulation, thereby contributing to a variety of clinical conditions. On the other hand, pathogens developed powerful evasion strategies to avoid complement attack by targeting the convertase. Finally, we increasingly challenge our bodies with foreign materials such as biomaterial implants or drug delivery vehicles that may induce adverse effects that are at least partially caused by complement activation and amplification via the alternative pathway. The involvement of the C3 convertase in a range of pathological conditions put this complex into the spotlight of complement-targeted drug discovery efforts VSports手机版. Fortunately, the physiological regulation and microbial evasion approaches provide a rich source of inspiration for the development of powerful treatment options. This review provides insight into the current knowledge about the molecular mechanisms that drive C3 convertase activity, reveals common and divergent strategies of convertase inhibition employed by host and pathogens, and how this inhibitory arsenal can be tapped for developing therapeutic options to treat complement-related diseases. .
Copyright © 2012 Elsevier GmbH. All rights reserved V体育安卓版. .
"V体育安卓版" Figures

References
-
- Blom AM, Hallstrom T, Riesbeck K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol. 2009;46:2808–2817. - PubMed
-
- Botto M, Kirschfink M, Macor P, Pickering MC, Wurzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol Immunol. 2009;46:2774–2783. - PubMed
-
- Bray BL. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat Rev Drug Discov. 2003;2:587–593. - "V体育2025版" PubMed
-
- Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol. 2009;69:479–491. - PubMed
-
- Chen H, Ricklin D, Hammel M, Garcia BL, McWhorter WJ, Sfyroera G, Wu YQ, Tzekou A, Li S, Geisbrecht BV, Woods VL, Jr, Lambris JD. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc Natl Acad Sci U S A. 2010;107:17621–17626. - PMC (V体育官网入口) - PubMed
Publication types
- Actions (VSports)
MeSH terms
- "VSports在线直播" Actions
- "V体育2025版" Actions
- Actions (VSports注册入口)
- Actions (V体育2025版)
- Actions (V体育安卓版)
- Actions (V体育官网)
- "V体育平台登录" Actions
"V体育安卓版" Substances
- V体育平台登录 - Actions
"V体育ios版" Grants and funding
V体育平台登录 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

