TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation
- PMID: 22921120
- PMCID: PMC3428731
- DOI: 10.1016/j.immuni.2012.04.015
TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation
Abstract (V体育官网)
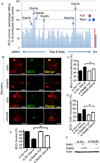
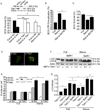
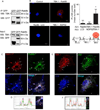
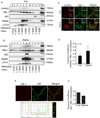
Autophagy is a fundamental biological process of the eukaryotic cell contributing to diverse cellular and physiological functions including cell-autonomous defense against intracellular pathogens. Here, we screened the Rab family of membrane trafficking regulators for effects on autophagic elimination of Mycobacterium tuberculosis var. bovis BCG and found that Rab8b and its downstream interacting partner, innate immunity regulator TBK-1, are required for autophagic elimination of mycobacteria in macrophages VSports手机版. TBK-1 was necessary for autophagic maturation. TBK-1 coordinated assembly and function of the autophagic machinery and phosphorylated the autophagic adaptor p62 (sequestosome 1) on Ser-403, a residue essential for its role in autophagic clearance. A key proinflammatory cytokine, IL-1β, induced autophagy leading to autophagic killing of mycobacteria in macrophages, and this IL-1β activity was dependent on TBK-1. Thus, TBK-1 is a key regulator of immunological autophagy and is responsible for the maturation of autophagosomes into lytic bactericidal organelles. .
Copyright © 2012 Elsevier Inc. All rights reserved V体育安卓版. .
Figures


"VSports手机版" References
-
- Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104:6031–6036. - "V体育官网入口" PMC - PubMed
-
- Clark K, Plater L, Peggie M, Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem. 2009;284:14136–14146. - VSports最新版本 - PMC - PubMed
VSports最新版本 - Publication types
- VSports在线直播 - Actions
MeSH terms
- "VSports注册入口" Actions
- Actions (V体育ios版)
- V体育安卓版 - Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- Actions (V体育平台登录)
- "V体育安卓版" Actions
- V体育官网 - Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- "VSports注册入口" Actions
- Actions (VSports手机版)
- VSports - Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- Actions (V体育安卓版)
- Actions (V体育ios版)
Substances (V体育官网入口)
- VSports最新版本 - Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育2025版 - Molecular Biology Databases
Miscellaneous

