Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells
- PMID: 22860037
- PMCID: PMC3409216
- DOI: 10.1371/journal.pone.0041945
Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells
Abstract
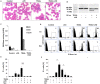
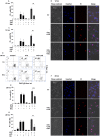
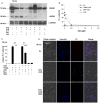
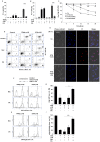
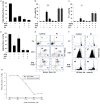
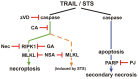
For a long time necrosis was thought to be an uncontrolled process but evidences recently have revealed that necrosis can also occur in a regulated manner. Necroptosis, a type of programmed necrosis is defined as a death receptor-initiated process under caspase-compromised conditions. The process requires the kinase activity of receptor-interacting protein kinase 1 and 3 (RIPK1 and RIPK3) and mixed lineage kinase domain-like protein (MLKL), as a substrate of RIPK3. The further downstream events remain elusive. We applied known inhibitors to characterize the contributing enzymes in necroptosis and their effect on cell viability and different cellular functions were detected mainly by flow cytometry VSports手机版. Here we report that staurosporine, the classical inducer of intrinsic apoptotic pathway can induce necroptosis under caspase-compromised conditions in U937 cell line. This process could be hampered at least partially by the RIPK1 inhibitor necrotstin-1 and by the heat shock protein 90 kDa inhibitor geldanamycin. Moreover both the staurosporine-triggered and the classical death ligand-induced necroptotic pathway can be effectively arrested by a lysosomal enzyme inhibitor CA-074-OMe and the recently discovered MLKL inhibitor necrosulfonamide. We also confirmed that the enzymatic role of poly(ADP-ribose)polymerase (PARP) is dispensable in necroptosis but it contributes to membrane disruption in secondary necrosis. In conclusion, we identified a novel way of necroptosis induction that can facilitate our understanding of the molecular mechanisms of necroptosis. Our results shed light on alternative application of staurosporine, as a possible anticancer therapeutic agent. Furthermore, we showed that the CA-074-OMe has a target in the signaling pathway leading to necroptosis. Finally, we could differentiate necroptotic and secondary necrotic processes based on participation of PARP enzyme. .
Conflict of interest statement
Competing Interests: The authors have read the journal's policy and have the following conflicts: IP is an employee of KPS Medical Biotechnology and Healthcare Services Ltd. KPS Medical Biotechnology did not support our studies at all. KPS Medical Biotechnology provides molecular pathology services to detect predictive biomarkers in tumor samples for personalized targeted therapies V体育安卓版. The company focuses on the diseases of the gastrointestinal tract and on lung cancer. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
References
-
- Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, et al. (2006) Cell death in pancreatitis: Caspases protect from necrotizing pancreatitis. Journal of Biological Chemistry 281: 3370–3381. - V体育平台登录 - PubMed
-
- McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S (2004) Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. American Journal of Physiology - Heart and Circulatory Physiology 286: H1923–H1935. - "V体育ios版" PubMed
-
- West T, Atzeva M, Holtzman DM (2006) Caspase-3 deficiency during development increases vulnerability to hypoxic-ischemic injury through caspase-3-independent pathways. Neurobiology of Disease 22: 523–537. - "VSports" PubMed
Publication types
MeSH terms
- Actions (V体育官网)
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- Actions (VSports)
- Actions (VSports注册入口)
Substances
- V体育官网 - Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

