Human TSC-associated renal angiomyolipoma cells are hypersensitive to ER stress
- PMID: 22791333
- PMCID: PMC3468523
- DOI: 10.1152/ajprenal.00441.2011
Human TSC-associated renal angiomyolipoma cells are hypersensitive to ER stress
"V体育安卓版" Abstract
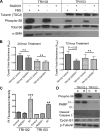
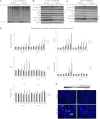
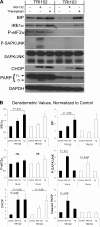
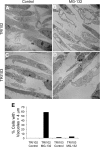
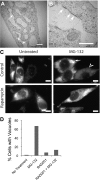
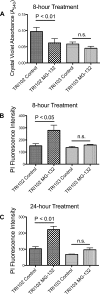
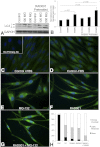
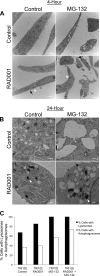
Tuberous sclerosis complex (TSC), an inherited tumor predisposition syndrome associated with mutations in TSC1 or TSC2, affects ∼1 in 6,000 individuals. Eighty percent of TSC patients develop renal angiomyolipomas, and renal involvement is a major contributor to patient morbidity and mortality. Recent work has shown that mammalian target of rapamycin complex 1 (mTORC1) inhibition caused angiomyolipoma shrinkage but that this treatment may cause cytostatic not a cytotoxic effect. Endoplasmic reticulum (ER) stress can develop in TSC-associated cells due to mTORC1-driven protein translation. We hypothesized that renal angiomyolipoma cells experience ER stress that can be leveraged to result in targeted cytotoxicity VSports手机版. We used immortalized human angiomyolipoma cells stably transfected with empty vector or TSC2 (encoding tuberin). Using cell number quantification and cell death assays, we found that mTORC1 inhibition with RAD001 suppressed angiomyolipoma cell proliferation in a cytostatic manner. Angiomyolipoma cells exhibited enhanced sensitivity to proteasome inhibitor-induced ER stress compared with TSC2-rescued cells. After proteasome inhibition with MG-132, Western blot analyses showed greater induction of C/EBP-homologous protein (CHOP) and more poly (ADP-ribose) polymerase (PARP) and caspase-3 cleavage, supporting ER stress-induced apoptosis. Live cell numbers also were decreased and cell death increased by MG-132 in angiomyolipoma cells compared with TSC2 rescued. Intriguingly, while pretreatment of angiomyolipoma cells with RAD001 attenuated CHOP and BiP induction, apoptotic markers cleaved PARP and caspase-3 and eukaryotic translation initiation factor 2α phosphorylation were increased, along with evidence of increased autophagy. These results suggest that human angiomyolipoma cells are uniquely susceptible to agents that exacerbate ER stress and that additional synergy may be achievable with targeted combination therapy. .
Figures




References
-
- Babcock JT, Quilliam LA. Rheb/mTOR activation and regulation in cancer: novel treatment strategies beyond rapamycin. Curr Drug Targets. - PubMed
-
- Catley L, Weisberg E, Kiziltepe T, Tai YT, Hideshima T, Neri P, Tassone P, Atadja P, Chauhan D, Munshi NC, Anderson KC. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 108: 3441–3449, 2006 - PMC - PubMed
-
- Cocciolone RA, Crotty KA, Andrews L, Haass NK, Moloney FJ. Multiple desmoplastic melanomas in Birt-Hogg-Dube syndrome and a proposed signaling link between folliculin, the mTOR pathway, and melanoma susceptibility. Arch Dermatol 146: 1316–1318 - PubMed (VSports)
-
- Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, Davies FE. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 110: 2641–2649, 2007 - "VSports在线直播" PubMed
Publication types
- VSports app下载 - Actions
- "V体育ios版" Actions
MeSH terms
- "V体育ios版" Actions
- "V体育安卓版" Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
- Actions (VSports app下载)
- V体育2025版 - Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- VSports app下载 - Actions
- VSports - Actions
- "VSports app下载" Actions
- "VSports app下载" Actions
"V体育官网" Substances
- V体育官网入口 - Actions
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- Actions (VSports注册入口)
- "VSports最新版本" Actions
- VSports app下载 - Actions
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

