V体育平台登录 - Disruption of the VDAC2-Bak interaction by Bcl-x(S) mediates efficient induction of apoptosis in melanoma cells
- PMID: 22705850
- PMCID: PMC3504705
- DOI: 10.1038/cdd.2012.71
Disruption of the VDAC2-Bak interaction by Bcl-x(S) mediates efficient induction of apoptosis in melanoma cells
Abstract
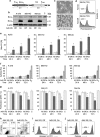
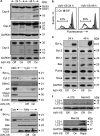
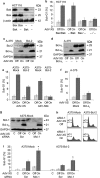
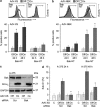
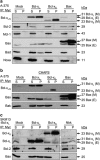
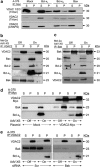
The proapoptotic B-cell lymphoma (Bcl)-2 protein Bcl-x(S) encloses the Bcl-2 homology (BH) domains BH3 and BH4 and triggers apoptosis via the multidomain protein Bak, however, the mechanism remained elusive. For investigating Bcl-x(S) efficacy and pathways, an adenoviral vector was constructed with its cDNA under tetracycline-off control. Bcl-x(S) overexpression resulted in efficient apoptosis induction and caspase activation in melanoma cells. Indicative of mitochondrial apoptosis pathways, Bcl-x(S) translocated to the mitochondria, disrupted the mitochondrial membrane potential and induced release of cytochrome c, apoptosis-inducing factor and second mitochondria-derived activator of caspases. In melanoma cells, Bcl-x(S) resulted in significant Bak activation, and Bak knockdown as well as Bcl-x(L) overexpression abrogated Bcl-x(S)-induced apoptosis, whereas Mcl-1 (myeloid cell leukemia-1) knockdown resulted in a sensitization. With regard to the particular role of voltage-dependent anion channel 2 (VDAC2) for inhibition of Bak, we identified here a notable interaction between Bcl-x(S) and VDAC2 in melanoma cells, which was proven in reciprocal coimmunoprecipitation analyses. On the other hand, Bcl-x(S) showed no direct interaction with Bak, and its binding to VDAC2 appeared as also independent of Bak expression. Suggesting a new proapoptotic mechanism, Bcl-x(S) overexpression resulted in disruption of the VDAC2-Bak interaction leading to release of Bak. Further supporting this pathway, overexpression of VDAC2 strongly decreased apoptosis by Bcl-x(S) VSports手机版. New proapoptotic pathways are of principle interest for overcoming apoptosis deficiency of melanoma cells. .
Figures

References
-
- Eberle J, Kurbanov BM, Hossini AM, Trefzer U, Fecker LF. Overcoming apoptosis deficiency of melanoma-hope for new therapeutic approaches. Drug Resist Updat. 2007;10:218–234. - PubMed
-
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. - "VSports最新版本" PubMed
-
- Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. - PubMed
-
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. - PubMed
-
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. - PubMed
"V体育官网入口" Publication types
- "VSports最新版本" Actions
V体育平台登录 - MeSH terms
- "V体育官网" Actions
- V体育2025版 - Actions
- "VSports最新版本" Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- "VSports手机版" Actions
- "V体育2025版" Actions
- VSports手机版 - Actions
- Actions (V体育平台登录)
- "VSports" Actions
- V体育官网 - Actions
Substances
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- "VSports app下载" Actions
"VSports app下载" LinkOut - more resources
Full Text Sources
Research Materials

