NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis
- PMID: 22699511
- PMCID: "V体育平台登录" PMC3387125
- DOI: "V体育平台登录" 10.1073/pnas.1201836109
NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis
Abstract
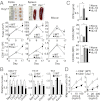
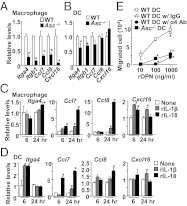
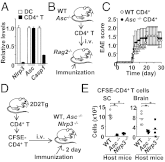
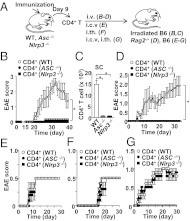
The NLRP3 inflammasome is a multiprotein complex consisting of three kinds of proteins, NLRP3, ASC, and pro-caspase-1, and plays a role in sensing pathogens and danger signals in the innate immune system. The NLRP3 inflammasome is thought to be involved in the development of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). However, the mechanism by which the NLRP3 inflammasome induces EAE is not clear VSports手机版. In this study, we found that the NLRP3 inflammasome played a critical role in inducing T-helper cell migration into the CNS. To gain migratory ability, CD4(+) T cells need to be primed by NLRP3 inflammasome-sufficient antigen-presenting cells to up-regulate chemotaxis-related proteins, such as osteopontin, CCR2, and CXCR6. In the presence of the NLRP3 inflammasome, dendritic cells and macrophages also induce chemotactic ability and up-regulate chemotaxis-related proteins, such as α4β1 integrin, CCL7, CCL8, and CXCL16. On the other hand, reduced Th17 cell population size in immunized Nlrp3(-/-) and Asc(-/-) mice is not a determinative factor for their resistance to EAE. As currently applied in clinical interventions of MS, targeting immune cell migration molecules may be an effective approach in treating MS accompanied by NLRP3 inflammasome activation. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures


"VSports app下载" References
-
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. - "VSports" PMC - PubMed
-
- Brodmerkel CM, et al. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005;175:5370–5378. - PubMed
-
- dos Santos AC, et al. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis—An intravital microscopy study. J Neuroimmunol. 2005;162:122–129. - "V体育2025版" PubMed
-
- Kataoka H, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–448. - PubMed
-
- Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63–66. - PubMed
Publication types
VSports注册入口 - MeSH terms
- VSports最新版本 - Actions
- V体育2025版 - Actions
- "VSports app下载" Actions
- Actions (VSports最新版本)
- "V体育官网入口" Actions
- VSports最新版本 - Actions
Substances
- Actions (VSports)
Grants and funding
LinkOut - more resources
"V体育官网" Full Text Sources
Other Literature Sources
Molecular Biology Databases
"VSports app下载" Research Materials
Miscellaneous

