Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease
- PMID: 22679138
- PMCID: PMC3427736
- DOI: V体育平台登录 - 10.1161/CIRCRESAHA.111.259960
Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease
Abstract
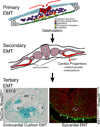
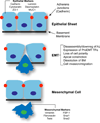
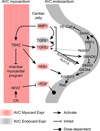
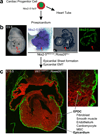
Epithelial to mesenchymal transition (EMT) converts epithelial cells to mobile and developmentally plastic mesenchymal cells. All cells in the heart arise from one or more EMTs. Endocardial and epicardial EMTs produce most of the noncardiomyocyte lineages of the mature heart VSports手机版. Endocardial EMT generates valve progenitor cells and is necessary for formation of the cardiac valves and for complete cardiac septation. Epicardial EMT is required for myocardial growth and coronary vessel formation, and it generates cardiac fibroblasts, vascular smooth muscle cells, a subset of coronary endothelial cells, and possibly a subset of cardiomyocytes. Emerging studies suggest that these developmental mechanisms are redeployed in adult heart valve disease, in cardiac fibrosis, and in myocardial responses to ischemic injury. Redirection and amplification of disease-related EMTs offer potential new therapeutic strategies and approaches for treatment of heart disease. Here, we review the role and molecular regulation of endocardial and epicardial EMT in fetal heart development, and we summarize key literature implicating reactivation of endocardial and epicardial EMT in adult heart disease. .
Figures

References
-
- Perez-Pomares JM, Munoz-Chapuli R. Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in Metazoans. Anat Rec. 2002;268:343–351. - PubMed
-
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. - PubMed (VSports在线直播)
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - "V体育官网入口" PubMed
-
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. - PubMed
Publication types
- V体育官网入口 - Actions
MeSH terms
- "V体育安卓版" Actions
- "V体育官网入口" Actions
- VSports注册入口 - Actions
- "VSports注册入口" Actions
Grants and funding (V体育官网入口)
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育平台登录 - Medical

