"V体育官网入口" The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis
- PMID: 22632973
- PMCID: PMC3586339
- DOI: 10.1016/j.cell.2012.02.065
VSports - The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis
Erratum in
- Cell. 2012 Nov 9;151(4):913-4
-
The Skp2-SCF E3 Ligase Regulates Akt Ubiquitination, Glycolysis, Herceptin Sensitivity, and Tumorigenesis.Cell. 2012 Nov 9;151(4):913-914. doi: 10.1016/j.cell.2012.10.025. Epub 2012 Nov 8. Cell. 2012. PMID: 30360292 No abstract available.
V体育官网 - Abstract
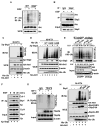
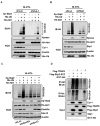
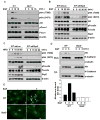
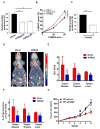
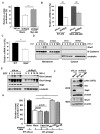
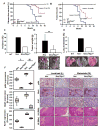
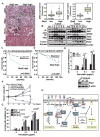
Akt kinase plays a central role in cell growth, metabolism, and tumorigenesis. The TRAF6 E3 ligase orchestrates IGF-1-mediated Akt ubiquitination and activation. Here, we show that Akt ubiquitination is also induced by activation of ErbB receptors; unexpectedly, and in contrast to IGF-1 induced activation, the Skp2 SCF complex, not TRAF6, is a critical E3 ligase for ErbB-receptor-mediated Akt ubiquitination and membrane recruitment in response to EGF VSports手机版. Skp2 deficiency impairs Akt activation, Glut1 expression, glucose uptake and glycolysis, and breast cancer progression in various tumor models. Moreover, Skp2 overexpression correlates with Akt activation and breast cancer metastasis and serves as a marker for poor prognosis in Her2-positive patients. Finally, Skp2 silencing sensitizes Her2-overexpressing tumors to Herceptin treatment. Our study suggests that distinct E3 ligases are utilized by diverse growth factors for Akt activation and that targeting glycolysis sensitizes Her2-positive tumors to Herceptin treatment. .
Copyright © 2012 Elsevier Inc V体育安卓版. All rights reserved. .
Figures
References
-
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. - PubMed
-
- Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. - V体育平台登录 - PubMed
-
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281–20286. - "VSports手机版" PubMed
-
- Birnbaum MJ. On the InterAktion between hexokinase and the mitochondrion. Dev Cell. 2004;7:781–782. - PubMed
-
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–25757. - PubMed
Publication types
MeSH terms (V体育官网)
- VSports手机版 - Actions
- "VSports" Actions
- "V体育官网" Actions
- "V体育官网入口" Actions
- "VSports" Actions
Substances
- "V体育官网入口" Actions
- V体育官网 - Actions
- Actions (VSports在线直播)
- VSports注册入口 - Actions
- V体育官网 - Actions
- Actions (V体育ios版)
"VSports注册入口" Grants and funding
LinkOut - more resources
Full Text Sources (VSports)
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
"V体育ios版" Miscellaneous

