Macrophage migration inhibitory factor enzymatic activity, lung inflammation, and cystic fibrosis
- PMID: 22592805
- PMCID: V体育平台登录 - PMC5448655
- DOI: 10.1164/rccm.201110-1864OC
Macrophage migration inhibitory factor enzymatic activity, lung inflammation, and cystic fibrosis
"V体育安卓版" Abstract
Rationale: Macrophage migration inhibitory factor (MIF) is a proinflammatory mediator with unique tautomerase enzymatic activity; the precise function has not been clearly defined. We previously demonstrated that individual patients with cystic fibrosis (CF) who are genetically predisposed to be high MIF producers develop accelerated end-organ injury VSports手机版. .
Objectives: To characterize the effects of the MIF-CATT polymorphism in patients with CF ex vivo. To investigate the role of MIF's tautomerase activity in a murine model of Pseudomonas aeruginosa infection V体育安卓版. .
Methods: MIF and tumor necrosis factor (TNF)-α protein levels were assessed in plasma or peripheral blood mononuclear cell (PBMC) supernatants by ELISA V体育ios版. A murine pulmonary model of chronic Pseudomonas infection was used in MIF wild-type mice (mif(+/+)) and in tautomerase-null, MIF gene knockin mice (mif (P1G/P1G)). .
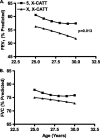
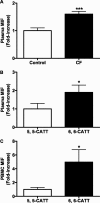
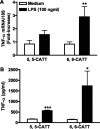
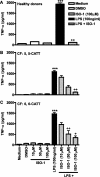
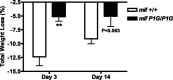
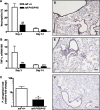
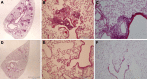
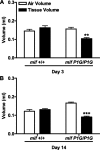
Measurements and main results: MIF protein was measured in plasma and PBMCs from 5- and 6-CATT patients with CF; LPS-induced TNF-α production from PBMCs was also assessed. The effect of a specific inhibitor of MIF-tautomerase activity, ISO-1, was investigated in PBMCs. In the murine infection model, total weight loss, differential cell counts, bacterial load, and intraacinar airspace/tissue volume were measured. MIF and TNF-α levels were increased in 6-CATT compared with 5-CATT patients with CF VSports最新版本. LPS-induced TNF-α production from PBMCs was attenuated in the presence of ISO-1. In a murine model of Pseudomonas infection, significantly less pulmonary inflammation and bacterial load was observed in mif(P1G/P1G) compared with mif(+/+) mice. .
Conclusions: MIF-tautomerase activity may provide a novel therapeutic target in patients with chronic inflammatory diseases such as CF, particularly those patients who are genetically predisposed to produce increased levels of this cytokine. V体育平台登录.
Figures
References
-
- Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of toll-like receptor 4. Nature 2001;414:920–924. - PubMed
-
- Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med 1997;3:320–323. - PubMed
-
- Morand EF, Leech M. Macrophage migration inhibitory factor in rheumatoid arthritis. Front Biosci 2005;10:12–22. - V体育2025版 - PubMed
Publication types
MeSH terms
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- Actions (V体育2025版)
- Actions (V体育ios版)
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- V体育官网 - Actions
- V体育ios版 - Actions
- VSports - Actions
- Actions (V体育官网)
- Actions (VSports在线直播)
- V体育ios版 - Actions
Substances
- VSports最新版本 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases (V体育官网入口)
"V体育安卓版" Miscellaneous

