Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator
- PMID: 22570484
- PMCID: PMC3381131
- DOI: 10.1074/jbc.M111.336537
Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator
VSports注册入口 - Abstract
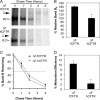
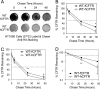
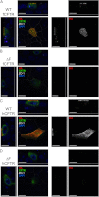
The most common cystic fibrosis transmembrane conductance regulator (CFTR) gene mutation is ΔF508, and this causes cystic fibrosis (CF). New CF models in the pig and ferret have been generated that develop lung, pancreatic, liver, and intestinal pathologies that reflect disease in CF patients. Species-specific biology in the processing of CFTR has demonstrated that pig and mouse ΔF508-CFTR proteins are more effectively processed to the apical membrane of airway epithelia than human ΔF508-CFTR. The processing behavior of ferret WT- and ΔF508-CFTR proteins remains unknown, and such information is important to predicting the utility of a ΔF508-CFTR ferret. To this end, we sought to compare processing, membrane stability, and function of human and ferret WT- and ΔF508-CFTR proteins in a heterologous expression system using HT1080, HEK293T, BHK21, and Cos7 cells as well as human and ferret CF polarized airway epithelia. Analysis of the protein processing and stability by metabolic pulse-chase and surface On-Cell Western blots revealed that WT-fCFTR half-life and membrane stability were increased relative to WT-hCFTR. Furthermore, in BHK21, Cos7, and CuFi cells, human and ferret ΔF508-CFTR processing was negligible, whereas low levels of processing of ΔF508-fCFTR could be seen in HT1080 and HEK293T cells. Only the WT-fCFTR, but not ΔF508-fCFTR, produced functional cAMP-inducible chloride currents in both CF human and ferret airway epithelia VSports手机版. Further elucidation of the mechanism responsible for elevated fCFTR protein stability may lead to new therapeutic approaches to augment CFTR function. These findings also suggest that generation of a ferret CFTR(ΔF508/ΔF508) animal model may be useful. .
Figures





References
-
- Rosenstein B. J., Zeitlin P. L. (1998) Cystic fibrosis. Lancet 351, 277–282 - PubMed
-
- Rowe S. M., Miller S., Sorscher E. J. (2005) Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 - "VSports app下载" PubMed
-
- Egan M. E. (2009) How useful are cystic fibrosis mouse models? Drug Discov. Today 6, 35–41
-
- Grubb B. R., Boucher R. C. (1999) Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol. Rev. 79, S193–S214 - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- VSports app下载 - Actions
- Actions (VSports)
- Actions (VSports app下载)
- Actions (VSports手机版)
- "VSports app下载" Actions
- Actions (V体育ios版)
- Actions (VSports最新版本)
"V体育官网" Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

