YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense
- PMID: 22563435
- PMCID: PMC3338577
- DOI: 10.1371/journal.pone.0036019
YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense (VSports)
Abstract
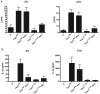
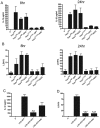
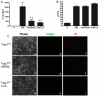
Yersinia outer protein J (YopJ) is a type III secretion system (T3SS) effector of pathogenic Yersinia (Yersinia pestis, Yersinia enterocolitica and Yersinia pseudotuberculosis) that is secreted into host cells. YopJ inhibits survival response pathways in macrophages, causing cell death. Allelic variation of YopJ is responsible for differential cytotoxicity in Yersinia strains. YopJ isoforms in Y. enterocolitica O:8 (YopP) and Y. pestis KIM (YopJ(KIM)) strains have high cytotoxic activity. In addition, YopJ(KIM)-induced macrophage death is associated with caspase-1 activation and interleukin-1β (IL-1β secretion VSports手机版. Here, the mechanism of YopJ(KIM)-induced cell death, caspase-1 activation, and IL-1β secretion in primary murine macrophages was examined. Caspase-3/7 activity was low and the caspase-3 substrate poly (ADP-ribose) polymerase (PARP) was not cleaved in Y. pestis KIM5-infected macrophages. In addition, cytotoxicity and IL-1β secretion were not reduced in the presence of a caspase-8 inhibitor, or in B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax)/Bcl-2 homologous antagonist/killer (Bak) knockout macrophages, showing that YopJ(KIM)-mediated cell death and caspase-1 activation occur independent of mitochondrial-directed apoptosis. KIM5-infected macrophages released high mobility group protein B1 (HMGB1), a marker of necrosis, and microscopic analysis revealed that necrotic cells contained active caspase-1, indicating that caspase-1 activation is associated with necrosis. Inhibitor studies showed that receptor interacting protein 1 (RIP1) kinase and reactive oxygen species (ROS) were not required for cytotoxicity or IL-β release in KIM5-infected macrophages. IL-1β secretion was reduced in the presence of cathepsin B inhibitors, suggesting that activation of caspase-1 requires cathepsin B activity. Ectopically-expressed YopP caused higher cytotoxicity and secretion of IL-1β in Y. pseudotuberculosis-infected macrophages than YopJ(KIM). Wild-type and congenic caspase 1 knockout C57BL/6 mice were equally susceptible to lethal infection with Y. pseudotuberculosis ectopically expressing YopP. These data suggest that YopJ-induced caspase-1 activation in Yersinia-infected macrophages is a downstream consequence of necrotic cell death and is dispensable for innate host resistance to a strain with enhanced cytotoxicity. .
Conflict of interest statement
"V体育平台登录" Figures




References
-
- Dockrell DH. Apoptotic cell death in the pathogenesis of infectious diseases. J Infect. 2001;42:227–234. - "VSports" PubMed
-
- Fairbairn IP. Macrophage apoptosis in host immunity to mycobacterial infections. Biochem Soc Trans. 2004;32:496–498. - PubMed
-
- Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000;2:265–273. - PubMed
-
- Porcelli SA, Jacobs WR., Jr Tuberculosis: unsealing the apoptotic envelope. Nat Immunol. 2008;9:1101–1102. - PubMed
Publication types (VSports)
MeSH terms
- V体育官网 - Actions
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- Actions (V体育平台登录)
- V体育安卓版 - Actions
- Actions (VSports手机版)
- "V体育平台登录" Actions
- "V体育2025版" Actions
- "V体育官网" Actions
- "VSports在线直播" Actions
- VSports手机版 - Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- Actions (VSports手机版)
- V体育安卓版 - Actions
- Actions (VSports)
- "VSports app下载" Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- Actions (V体育2025版)
- VSports app下载 - Actions
- Actions (V体育平台登录)
- Actions (VSports在线直播)
- VSports在线直播 - Actions
Substances
- "VSports" Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
- Actions (VSports手机版)
- "V体育ios版" Actions
- VSports注册入口 - Actions
- "V体育ios版" Actions
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources (VSports app下载)
Full Text Sources
Research Materials
Miscellaneous

