VSports - Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis
- PMID: 22549727
- PMCID: PMC3360561
- DOI: 10.1101/gad.187252.112
"V体育ios版" Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis
"VSports" Abstract
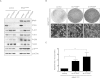
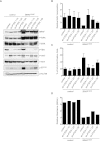
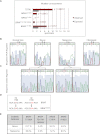
Human melanocytic nevi (moles) are benign lesions harboring activated oncogenes, including BRAF. Although this oncogene initially acts mitogenically, eventually, oncogene-induced senescence (OIS) ensues. Nevi can infrequently progress to melanomas, but the mechanistic relationship with OIS is unclear. We show here that PTEN depletion abrogates BRAF(V600E)-induced senescence in human fibroblasts and melanocytes. Correspondingly, in established murine BRAF(V600E)-driven nevi, acute shRNA-mediated depletion of PTEN prompted tumor progression. Furthermore, genetic analysis of laser-guided microdissected human contiguous nevus-melanoma specimens recurrently revealed identical mutations in BRAF or NRAS in adjacent benign and malignant melanocytes VSports手机版. The PI3K pathway was often activated through either decreased PTEN or increased AKT3 expression in melanomas relative to their adjacent nevi. Pharmacologic PI3K inhibition in melanoma cells suppressed proliferation and induced the senescence-associated tumor suppressor p15(INK4B). This treatment also eliminated subpopulations resistant to targeted BRAF(V600E) inhibition. Our findings suggest that a significant proportion of melanomas arise from nevi. Furthermore, these results demonstrate that PI3K pathway activation serves as a rate-limiting event in this setting, acting at least in part by abrogating OIS. The reactivation of senescence features and elimination of cells refractory to BRAF(V600E) inhibition by PI3K inhibition warrants further investigation into the therapeutic potential of simultaneously targeting these pathways in melanoma. .
Figures




References
-
- Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, Nazarian R, Chmielowski B, Glaspy JA, Comin-Anduix B, et al. 2011. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS ONE 6: e28973 doi: 10.1371/journal.pone.0028973 - V体育ios版 - PMC - PubMed
-
- Bauer J, Curtin JA, Pinkel D, Bastian BC 2007. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol 127: 179–182 - PubMed
-
- Bennett DC 2003. Human melanocyte senescence and melanoma susceptibility genes. Oncogene 22: 3063–3069 - PubMed
-
- Bevona C, Goggins W, Quinn T, Fullerton J, Tsao H 2003. Cutaneous melanomas associated with nevi. Arch Dermatol 139: 1620–1624 - VSports最新版本 - PubMed
Publication types
V体育安卓版 - MeSH terms
- Actions (V体育官网)
- "VSports" Actions
- Actions (V体育官网入口)
- V体育平台登录 - Actions
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- Actions (V体育2025版)
- Actions (V体育安卓版)
- V体育平台登录 - Actions
- Actions (VSports手机版)
- V体育平台登录 - Actions
- "VSports app下载" Actions
- VSports手机版 - Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- Actions (V体育2025版)
- V体育官网入口 - Actions
- "VSports app下载" Actions
- Actions (VSports最新版本)
- "V体育安卓版" Actions
Substances
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- V体育官网入口 - Actions
- "VSports" Actions
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous (V体育ios版)
