Cell-mediated autophagy promotes cancer cell survival
- PMID: 22505650
- PMCID: "V体育2025版" PMC3505669
- DOI: 10.1158/0008-5472.CAN-11-3396
Cell-mediated autophagy promotes cancer cell survival
Abstract (V体育安卓版)
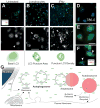
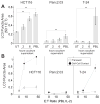
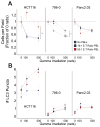
Immune effector cells integrate signals that define the nature and magnitude of the subsequent response. Experimental measures for immune cell-mediated lysis of tumors or virally infected targets rely on average responses of permeability or apoptotic changes within a population of targets. Here, we examined individual target cells following interaction with lymphoid effectors. We found that human peripheral blood lymphocytes not only provide lytic signals but also promote autophagy in the remaining cells. At high effector-to-target ratios, autophagy was induced in several human tumors, as assessed by induction of LC3 puncta and diminished p62 VSports手机版. Natural killer cells are a primary mediator of this process. In addition, target cell autophagy was enhanced by provision of interleukin (IL)-2, whereas IL-10 attenuated this effect, and cell-to-cell contact strongly enhanced lymphocyte-mediated autophagy. Although IFN-γ can induce autophagy in target cells, IFN-α acted directly on the targets or in concert with lymphocytes to diminish target autophagy in some cell types. Importantly, cell-mediated autophagy promoted resistance from treatment modalities designed to eradicate tumor cells. Our findings therefore show that the lymphocyte-induced cell-mediated autophagy promotes cancer cell survival and may represent an important target for development of novel therapies. .
"V体育2025版" Conflict of interest statement
Figures




V体育安卓版 - References
-
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–8. - "VSports app下载" PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. - PubMed
-
- Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36. - "V体育ios版" PubMed
Publication types
VSports注册入口 - MeSH terms
- "V体育2025版" Actions
- VSports注册入口 - Actions
- "VSports" Actions
- "V体育官网" Actions
- "VSports注册入口" Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
"VSports在线直播" Substances
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

