Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome
- PMID: 22479187
- PMCID: PMC3315489 (V体育官网)
- DOI: 10.1371/journal.ppat.1002638
Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome
Abstract
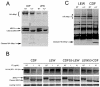
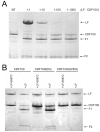
NOD-like receptor (NLR) proteins (Nlrps) are cytosolic sensors responsible for detection of pathogen and danger-associated molecular patterns through unknown mechanisms. Their activation in response to a wide range of intracellular danger signals leads to formation of the inflammasome, caspase-1 activation, rapid programmed cell death (pyroptosis) and maturation of IL-1β and IL-18. Anthrax lethal toxin (LT) induces the caspase-1-dependent pyroptosis of mouse and rat macrophages isolated from certain inbred rodent strains through activation of the NOD-like receptor (NLR) Nlrp1 inflammasome. Here we show that LT cleaves rat Nlrp1 and this cleavage is required for toxin-induced inflammasome activation, IL-1 β release, and macrophage pyroptosis. These results identify both a previously unrecognized mechanism of activation of an NLR and a new, physiologically relevant protein substrate of LT VSports手机版. .
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med. 2009;30:439–455. - "V体育2025版" PMC - PubMed
-
- Newman ZL, Printz MP, Liu S, Crown D, Breen L, et al. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 2010;6:e1000906. - "VSports" PMC - PubMed
-
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. - "VSports在线直播" PubMed
-
- Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J. 2000;352 Pt 3:739–745. - V体育安卓版 - PMC - PubMed
Publication types
MeSH terms
- Actions (VSports注册入口)
- Actions (V体育ios版)
- Actions (V体育2025版)
- "V体育官网" Actions
- Actions (V体育2025版)
- "V体育官网" Actions
- Actions (V体育安卓版)
Substances
- Actions (V体育官网入口)
- VSports注册入口 - Actions
VSports在线直播 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
VSports注册入口 - Miscellaneous

