"VSports" Oxidatively truncated phospholipids are required agents of tumor necrosis factor α (TNFα)-induced apoptosis
- PMID: 22433871
- PMCID: PMC3366783
- DOI: 10.1074/jbc.M111.300012
Oxidatively truncated phospholipids are required agents of tumor necrosis factor α (TNFα)-induced apoptosis
Abstract
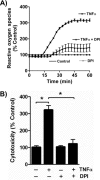
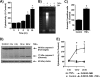
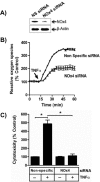
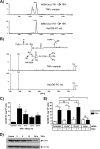
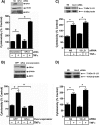
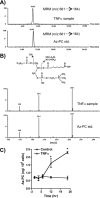
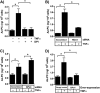
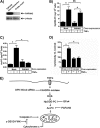
TNFα generates reactive oxygen species (ROS) at the cell surface that induce cell death, but how ROS communicate to mitochondria and their specific apoptotic action(s) are both undefined. ROS oxidize phospholipids to hydroperoxides that are friable and fragment adjacent to the (hydro)peroxide function, forming truncated phospholipids, such as azelaoyl phosphatidylcholine (Az-PC). Az-PC is relatively soluble, and exogenous Az-PC rapidly enters cells to damage mitochondrial integrity and initiate intrinsic apoptosis. We determined whether this toxic phospholipid is formed within cells during TNFα stimulation in sufficient quantities to induce apoptosis and if they are essential in TNFα-induced cytotoxicity. We found that TNFα induced ROS formation and phospholipid peroxidation in Jurkat cells, and either chemical interference with NADPH oxidase activity or siRNA suppression of the NADPH oxidase-4 subunit blocked ROS accumulation and phospholipid peroxidation. Mass spectrometry showed that phospholipid peroxides and then Az-PC increased after TNFα exposure, whereas ROS inhibition abolished Az-PC accumulation and TNFα-induced cell death. Glutathione peroxidase-4 (GPx4), which specifically metabolizes lipid hydroperoxides, fell in TNFα-stimulated cells prior to death VSports手机版. Ectopic GPx4 overcame this, reduced peroxidized phospholipid accumulation, blocked Az-PC accumulation, and prevented death. Conversely, GPx4 siRNA knockdown enhanced phospholipid peroxidation, increasing TNFα-stimulated Az-PC formation and apoptosis. Truncated phospholipids were essential elements of TNFα-induced apoptosis because overexpression of PAFAH2 (a phospholipase A(2) that selectively hydrolyzes truncated phospholipids) blocked TNFα-induced Az-PC accumulation without affecting phospholipid peroxidation. PAFAH2 also abolished apoptosis. Thus, phospholipid oxidation and truncation to apoptotic phospholipids comprise an essential element connecting TNFα receptor signaling to mitochondrial damage and apoptotic death. .
V体育安卓版 - Figures
References
-
- Gordon J. W., Shaw J. A., Kirshenbaum L. A. (2011) Multiple facets of NF-κB in the heart. To be or not to NF-κB. Circ. Res. 108, 1122–1132 - PubMed
-
- Falschlehner C., Emmerich C. H., Gerlach B., Walczak H. (2007) TRAIL signaling. Decisions between life and death. Int. J. Biochem. Cell Biol. 39, 1462–1475 - VSports注册入口 - PubMed
-
- Schafer Z. T., Kornbluth S. (2006) The apoptosome. Physiological, developmental, and pathological modes of regulation. Dev. Cell 10, 549–561 - PubMed
Publication types
MeSH terms
- Actions (V体育官网入口)
- "VSports" Actions
- Actions (V体育ios版)
- Actions (VSports)
- "VSports注册入口" Actions
- VSports在线直播 - Actions
Substances
- "V体育官网" Actions
- VSports - Actions
- Actions (VSports最新版本)
- V体育平台登录 - Actions
- Actions (VSports app下载)
- V体育官网入口 - Actions
Grants and funding
"V体育平台登录" LinkOut - more resources
"V体育ios版" Full Text Sources

