Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation
- PMID: 22425248
- PMCID: PMC3314246
- DOI: 10.1016/j.immuni.2012.01.015
Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation
Abstract
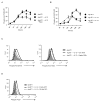
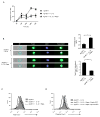
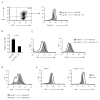
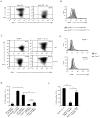
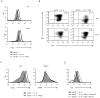
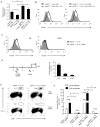
The evolutionary conserved Foxo transcription factors are important regulators of quiescence and longevity. Although, Foxo1 is known to be important in regulating CD8(+) T cell trafficking and homeostasis, its role in functional differentiation of antigen-stimulated CD8(+) T cells is unclear. Herein, we demonstrate that inactivation of Foxo1 was essential for instructing T-bet transcription factor-mediated effector differentiation of CD8(+) T cells. The Foxo1 inactivation was dependent on mTORC1 kinase, given that blockade of mTORC1 abrogated mTORC2-mediated Akt (Ser473) kinase phosphorylation, resulting in Foxo1-dependent switch from T-bet to Eomesodermin transcription factor activation and increase in memory precursors. Silencing Foxo1 ablated interleukin-12- and rapamycin-enhanced CD8(+) T cell memory responses and restored T-bet-mediated effector functions. These results demonstrate an essential role of Foxo1 in actively repressing effector or terminal differentiation processes to promote memory CD8(+) T cell development and identify the functionally diverse mechanisms utilized by Foxo1 to promote quiescence and longevity VSports手机版. .
Copyright © 2012 Elsevier Inc V体育安卓版. All rights reserved. .
VSports手机版 - Figures

"V体育安卓版" References
-
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. - V体育官网入口 - PMC - PubMed
-
- Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–2262. - PubMed
Publication types (V体育安卓版)
- "V体育2025版" Actions
MeSH terms
- Actions (V体育官网入口)
- VSports app下载 - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "V体育官网" Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- VSports app下载 - Actions
- "V体育官网" Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
- Actions (VSports最新版本)
- "V体育平台登录" Actions
- VSports app下载 - Actions
VSports在线直播 - Substances
- V体育2025版 - Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- Actions (VSports)
- Actions (VSports app下载)
- "VSports手机版" Actions
- V体育2025版 - Actions
Grants and funding
V体育平台登录 - LinkOut - more resources
V体育2025版 - Full Text Sources
Other Literature Sources
Molecular Biology Databases
"VSports在线直播" Research Materials
Miscellaneous

