Ipilimumab: its potential in non-small cell lung cancer (V体育官网入口)
- PMID: 22423263
- PMCID: PMC3296082
- DOI: 10.1177/1758834011431718
Ipilimumab: its potential in non-small cell lung cancer
"VSports注册入口" Abstract
Ipilimumab is a fully human monoclonal antibody that enhances antitumor immunity by way of cytotoxic T-lymphocyte antigen 4 blockade. It has already been approved by the US Food and Drug Administration for the treatment of metastatic melanoma and is being investigated for treating other solid tumors such as renal cell, prostate and lung cancers. This review details the potential of ipilimumab in the management of non-small cell lung cancer (NSCLC). In particular, ipilimumab showed promising results in a first-line NSCLC phase II study combining carboplatin/paclitaxel chemotherapy with concurrent or phased ipilimumab. The median immune-related progression-free survival was 5 VSports手机版. 68 months for the phased ipilimumab arm versus 4. 63 months for chemotherapy alone (hazard ratio [HR] = 0. 68, p = 0. 026) and 5. 52 months for the concurrent ipilimumab arm versus 4. 63 months for chemotherapy alone (HR = 0. 77, p = 0. 094). The main adverse events were immune related, such as hypophysitis, enterocolitis, and hyperthyroidism. These adverse events may be improved with high-dose glucocorticoids and may be correlated with tumor response. Phase III studies are ongoing. Future studies may investigate ipilimumab in the management of early stage lung cancer. Strategies for potential translational research studies are also discussed to identify prognostic and predictive biomarkers for the use of ipilimumab in the treatment of patients with NSCLC. .
Keywords: biomarkers; immunotherapy; ipilimumab; lung cancer; targeted therapy. V体育安卓版.
VSports app下载 - Conflict of interest statement
The authors have participated in clinical trials based on ipilimumab in lung cancer.
"V体育官网" Figures
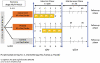
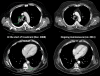
V体育ios版 - References
-
- Arriagada R., Dunant A., Pignon J.P., Bergman B., Chabowski M., Grunenwald D., et al. (2010) Long-term results of the International Adjuvant Lung Cancer Trial evaluating cisplatine-based chemotherapy in resected lung cancer. J Clin Oncol 28: 35–42 - "V体育安卓版" PubMed
-
- Bass K.K., Mastrangelo M.J. (1998) Immunopotentiation with low-dose cyclophosphamide in the active specific immumnotherapy of cancer. Cancer Immunol Immunother 47: 1–12 - PMC (V体育2025版) - PubMed
-
- Breunis W.B., Tarazona-Santos E., Chen R., Kiley M., Rosenberg S.A., Chanock S.J. (2008) Influence of cytotoxic T lymphocyte- associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J Immunother 31: 586–590 - PMC (VSports注册入口) - PubMed
"VSports手机版" LinkOut - more resources
Full Text Sources
Other Literature Sources

