Explaining calcium-dependent gating of anoctamin-1 chloride channels requires a revised topology
- PMID: 22394518
- PMCID: PMC3558997
- DOI: V体育安卓版 - 10.1161/CIRCRESAHA.112.264440
VSports最新版本 - Explaining calcium-dependent gating of anoctamin-1 chloride channels requires a revised topology
V体育ios版 - Abstract
Rationale: Ca2+ -activated Cl channels play pivotal roles in the cardiovascular system VSports手机版. They regulate vascular smooth muscle tone and participate in cardiac action potential repolarization in some species. Ca2+ -activated Cl channels were recently discovered to be encoded by members of the anoctamin (Ano, also called Tmem16) superfamily, but the mechanisms of Ano1 gating by Ca2+ remain enigmatic. .
Objective: The objective was to identify regions of Ano1 involved in channel gating by Ca2+ V体育安卓版. .
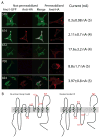
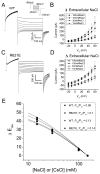
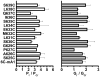
Methods and results: The Ca2+ sensitivity of Ano1 was estimated from rates of current activation, and deactivation in excised patches rapidly switched between zero and high Ca2+ on the cytoplasmic side. Mutation of glutamates E702 and E705 dramatically altered Ca2+ sensitivity. E702 and E705 are predicted to be in an extracellular loop, but antigenic epitopes introduced into this loop are not accessible to extracellular antibodies, suggesting this loop is intracellular. Cytoplasmically applied membrane-impermeant sulfhydryl reagents alter the Ca2+ sensitivity of Ano1 E702C and E705C as expected if E702 and E705 are intracellular. Substituted cysteine accessibility mutagenesis of the putative re-entrant loop suggests that E702 and E705 are located adjacent to the Cl conduction pathway V体育ios版. .
Conclusions: We propose an alternative model of Ano1 topology based on mutagenesis, epitope accessibility, and cysteine-scanning accessibility. These data contradict the popular re-entrant loop model by showing that the putative fourth extracellular loop (ECL 4) is intracellular and may contain a Ca2+ binding site VSports最新版本. These studies provide new perspectives on regulation of Ano1 by Ca2+. .
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures




References
-
- Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: A complex picture is emerging. Can J Physiol Pharmacol. 2005;83:541–556. - "VSports app下载" PubMed
-
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–454. - PubMed
-
- Duan D. Phenomics of cardiac chloride channels: The systematic study of chloride channel function in the heart. J Physiol. 2009;587:2163–2177. - "V体育官网入口" PMC - PubMed
-
- Gwanyanya A, Macianskiene R, Bito V, Sipido KR, Vereecke J, Mubagwa K. Inhibition of the calcium-activated chloride current in cardiac ventricular myocytes by n-(p-amylcinnamoyl)anthranilic acid (ACA) Biochemical and Biophysical Research Communications. 2010;402:531–536. - "VSports app下载" PubMed
Publication types
MeSH terms
- "V体育官网" Actions
- Actions (V体育官网)
- "V体育安卓版" Actions
- "V体育2025版" Actions
- VSports在线直播 - Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- "VSports" Actions
- V体育ios版 - Actions
Substances
- Actions (V体育平台登录)
Grants and funding
V体育ios版 - LinkOut - more resources
"VSports在线直播" Full Text Sources
Miscellaneous

