Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma
- PMID: 22374334
- PMCID: PMC4607283
- DOI: 10.1158/1078-0432.CCR-11-2413
Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma
Erratum in
-
Correction: Aurora A Inhibitor (MLN8237) plus Vincristine plus Rituximab Is Synthetic Lethal and a Potential Curative Therapy in Aggressive B-cell Non-Hodgkin Lymphoma.Clin Cancer Res. 2015 Nov 15;21(22):5181. doi: 10.1158/1078-0432.CCR-15-2382. Clin Cancer Res. 2015. PMID: 26567366 No abstract available.
"VSports app下载" Abstract
Purpose: Aurora A and B are oncogenic serine/threonine kinases that regulate mitosis. Overexpression of Auroras promotes resistance to microtubule-targeted agents. We investigated mechanistic synergy by inhibiting the mitotic spindle apparatus in the presence of MLN8237 [M], an Aurora A inhibitor with either vincristine [MV] or docetaxel [MD] in aggressive B-cell non-Hodgkin lymphoma (B-NHL). The addition of rituximab [R] to MV or MD was evaluated for synthetic lethality. VSports手机版.
Experimental design: Aggressive B-NHL cell subtypes were evaluated in vitro and in vivo for target modulation and anti-NHL activity with single agents, doublets, and triplets by analyzing cell proliferation, apoptosis, tumor growth, survival, and mechanisms of response/relapse by gene expression profiling with protein validation V体育安卓版. .
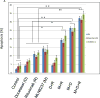
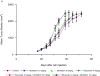
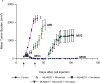
Results: MV is synergistic whereas MD is additive for cell proliferation inhibition in B-NHL cell culture models. Addition of rituximab to MV is superior to MD, but both significantly induce apoptosis compared with doublet therapy. Mouse xenograft models of mantle cell lymphoma showed modest single-agent activity for MLN8237, rituximab, docetaxel, and vincristine with tumor growth inhibition (TGI) of approximately 10% to 15% V体育ios版. Of the doublets, MV caused tumor regression, whereas TGI was observed with MD (approximately 55%-60%) and MR (approximately 25%-50%), respectively. Although MV caused tumor regression, mice relapsed 20 days after stopping therapy. In contrast, MVR was curative, whereas MDR led to TGI of approximately 85%. Proliferation cell nuclear antigen, Aurora B, cyclin B1, cyclin D1, and Bcl-2 proteins of harvested tumors confirmed response and resistance to therapy. .
Conclusions: Addition of rituximab to MV is a novel therapeutic strategy for aggressive B-NHL and warrants clinical trial evaluation VSports最新版本. .
©2012 AACR.
Conflict of interest statement
The authors declare no competing financial interests.
"VSports" Figures









References
-
- Green MR, Woolery JE, Mahadevan D. Update on Aurora Kinase Targeted Therapeutics in Oncology. Expert Opin Drug Discov. 2011;6(3):291–307. - PMC (V体育安卓版) - PubMed
-
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–66. - PubMed
-
- Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. - V体育平台登录 - PubMed
-
- Arbitrario JP, Belmont BJ, Evanchik MJ, Flanagan WM, Fucini RV, Hansen SK, et al. SNS-314, a pan-Aurora kinase inhibitor, shows potent anti-tumor activity and dosing flexibility in vivo. Cancer Chemother Pharmacol. 65:707–17. - PubMed (VSports在线直播)
Publication types
- VSports app下载 - Actions
VSports手机版 - MeSH terms
- VSports注册入口 - Actions
- Actions (V体育平台登录)
- Actions (VSports app下载)
- "V体育ios版" Actions
- "V体育2025版" Actions
- VSports最新版本 - Actions
- Actions (VSports在线直播)
- VSports - Actions
- "VSports" Actions
- "VSports app下载" Actions
- VSports - Actions
- "V体育ios版" Actions
- Actions (VSports手机版)
- V体育安卓版 - Actions
- Actions (VSports)
- Actions (VSports手机版)
- Actions (VSports注册入口)
- "V体育平台登录" Actions
- "VSports手机版" Actions
Substances (V体育ios版)
- VSports手机版 - Actions
- Actions (V体育ios版)
- Actions (V体育ios版)
- Actions (V体育平台登录)
- Actions (VSports手机版)
- Actions (V体育平台登录)
- Actions (V体育官网)
- VSports最新版本 - Actions
- "VSports在线直播" Actions
- VSports注册入口 - Actions
Grants and funding
V体育安卓版 - LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

