Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells
- PMID: 22371374
- PMCID: PMC3347451
- DOI: 10.1128/IAI.00050-12
Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells
Abstract
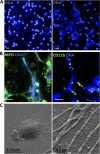
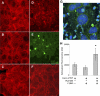
We recently documented the neutrophil response to enterovirulent diffusely adherent Escherichia coli expressing Afa/Dr fimbriae (Afa/Dr DAEC), using the human myeloid cell line PLB-985 differentiated into fully mature neutrophils. Upon activation, particularly during infections, neutrophils release neutrophil extracellular traps (NETs), composed of a nuclear DNA backbone associated with antimicrobial peptides, histones, and proteases, which entrap and kill pathogens. Here, using fluorescence microscopy and field emission scanning electron microscopy, we observed NET production by PLB-985 cells infected with the Afa/Dr wild-type (WT) E. coli strain C1845 VSports手机版. We found that these NETs were able to capture, immobilize, and kill WT C1845 bacteria. We also developed a coculture model of human enterocyte-like Caco-2/TC7 cells and PLB-985 cells previously treated with WT C1845 and found, for the first time, that the F-actin cytoskeleton of enterocyte-like cells is damaged in the presence of bacterium-induced NETs and that this deleterious effect is prevented by inhibition of protease release. These findings provide new insights into the neutrophil response to bacterial infection via the production of bactericidal NETs and suggest that NETs may damage the intestinal epithelium, particularly in situations such as inflammatory bowel diseases. .
"VSports手机版" Figures
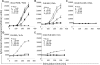

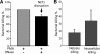
References
-
- Berger CN, Billker O, Meyer TF, Servin AL, Kansau I. 2004. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol. Microbiol. 52:963–983 - PubMed
-
- Bernet-Camard MF, Coconnier MH, Hudault S, Servin AL. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918–1928 - PMC - PubMed
-
- Betis F, et al. 2003. Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins. Infect. Immun. 71:1774–1783 - PMC (VSports手机版) - PubMed
MeSH terms
- "VSports" Actions
- Actions (VSports在线直播)
- Actions (V体育官网入口)
- Actions (VSports最新版本)
- "V体育ios版" Actions
- V体育安卓版 - Actions
Substances
- "V体育安卓版" Actions
- VSports - Actions
- "VSports最新版本" Actions
LinkOut - more resources
Full Text Sources

