VSports - Repression of Salmonella enterica phoP expression by small molecules from physiological bile
- PMID: 22366421
- PMCID: PMC3347055 (V体育ios版)
- DOI: 10.1128/JB.00104-12
Repression of Salmonella enterica phoP expression by small molecules from physiological bile
Abstract
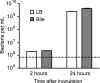
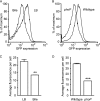
Infection with Salmonella enterica serovar Typhi in humans causes the life-threatening disease typhoid fever. In the laboratory, typhoid fever can be modeled through the inoculation of susceptible mice with Salmonella enterica serovar Typhimurium. Using this murine model, we previously characterized the interactions between Salmonella Typhimurium and host cells in the gallbladder and showed that this pathogen can successfully invade gallbladder epithelial cells and proliferate. Additionally, we showed that Salmonella Typhimurium can use bile phospholipids to grow at high rates. These abilities are likely important for quick colonization of the gallbladder during typhoid fever and further pathogen dissemination through fecal shedding. To further characterize the interactions between Salmonella and the gallbladder environment, we compared the transcriptomes of Salmonella cultures grown in LB broth or physiological murine bile. Our data showed that many genes involved in bacterial central metabolism are affected by bile, with the citric acid cycle being repressed and alternative respiratory systems being activated. Additionally, our study revealed a new aspect of Salmonella interactions with bile through the identification of the global regulator phoP as a bile-responsive gene VSports手机版. Repression of phoP expression could also be achieved using physiological, but not commercial, bovine bile. The biological activity does not involve PhoPQ sensing of a bile component and is not caused by bile acids, the most abundant organic components of bile. Bioactivity-guided purification allowed the identification of a subset of small molecules from bile that can elicit full activity; however, a single compound with phoP inhibitory activity could not be isolated, suggesting that multiple molecules may act in synergy to achieve this effect. Due to the critical role of phoP in Salmonella virulence, further studies in this area will likely reveal aspects of the interaction between Salmonella and bile that are relevant to disease. .
Figures
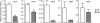
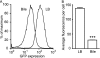
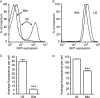

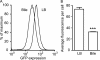
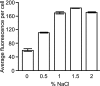
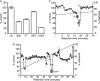
References
"VSports app下载" Publication types
- "VSports在线直播" Actions
MeSH terms
- Actions (VSports在线直播)
- "V体育安卓版" Actions
- "V体育ios版" Actions
- "VSports" Actions
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- VSports - Actions
- V体育官网 - Actions
Substances
- "VSports" Actions
- Actions (VSports)
Grants and funding
LinkOut - more resources
Full Text Sources (V体育2025版)
Molecular Biology Databases

