Discovery of a cytosolic/soluble ferroxidase in rodent enterocytes
- PMID: 22331876
- PMCID: PMC3295312
- DOI: 10.1073/pnas.1120833109
Discovery of a cytosolic/soluble ferroxidase in rodent enterocytes
Abstract
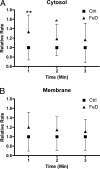
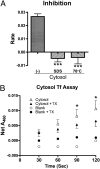
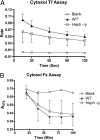
Hephaestin (Heph), a membrane-bound multicopper ferroxidase (FOX) expressed in duodenal enterocytes, is required for optimal iron absorption. However, sex-linked anemia (sla) mice harboring a 194-amino acid deletion in the Heph protein are able to absorb dietary iron despite reduced expression and mislocalization of the mutant protein. Thus Heph may not be essential, and mice are able to compensate for the loss of its activity VSports手机版. The current studies were undertaken to search for undiscovered FOXs in rodent enterocytes. An experimental approach was developed to investigate intestinal FOXs in which separate membrane and cytosolic fractions were prepared and FOX activity was measured by a spectrophotometric transferrin-coupled assay. Unexpectedly, FOX activity was noted in membrane and cytosolic fractions of rat enterocytes. Different experimental approaches demonstrated that cytosolic FOX activity was not caused by contamination with membrane Heph or a method-induced artifact. Cytosolic FOX activity was abolished by SDS and heat (78 °C), suggesting protein-mediated iron oxidation, and was also sensitive to Triton X-100. Furthermore, cytosolic FOX activity increased ∼30% in iron-deficient rats (compared with controls) but was unchanged in copper-deficient rats (in contrast to the reported dramatic reduction of Heph expression and activity during copper deficiency). Additional studies done in sla, Heph-knockout, and ceruloplasmin-knockout mice proved that cytosolic FOX activity could not be fully explained by Heph or ceruloplasmin. Therefore rodent enterocytes contain a previously undescribed soluble cytosolic FOX that may function in transepithelial iron transport and complement membrane-bound Heph. .
"V体育ios版" Conflict of interest statement
The authors declare no conflict of interest.
Figures (V体育ios版)



References
-
- Chen H, et al. Systemic regulation of Hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood. 2003;102:1893–1899. - PubMed
-
- Hudson DM, et al. Human hephaestin expression is not limited to enterocytes of the gastrointestinal tract but is also found in the antrum, the enteric nervous system, and pancreatic β-cells. Am J Physiol Gastrointest Liver Physiol. 2010;298:G425–G432. - PubMed (V体育平台登录)
-
- Chen H, et al. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood. 2004;103:3933–3939. - PubMed
-
- Cherukuri S, et al. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2:309–319. - PubMed
"V体育平台登录" Publication types
- Actions (V体育2025版)
MeSH terms
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- "V体育平台登录" Actions
- "V体育官网" Actions
- V体育2025版 - Actions
- Actions (VSports)
- "VSports" Actions
- "VSports手机版" Actions
- V体育官网入口 - Actions
- Actions (V体育官网)
- Actions (VSports手机版)
- "V体育官网入口" Actions
- Actions (V体育2025版)
- "V体育官网入口" Actions
Substances
- Actions (VSports手机版)
- V体育官网入口 - Actions
Supplementary concepts (V体育ios版)
Grants and funding
LinkOut - more resources (V体育ios版)
Full Text Sources (V体育官网)
"VSports最新版本" Research Materials

